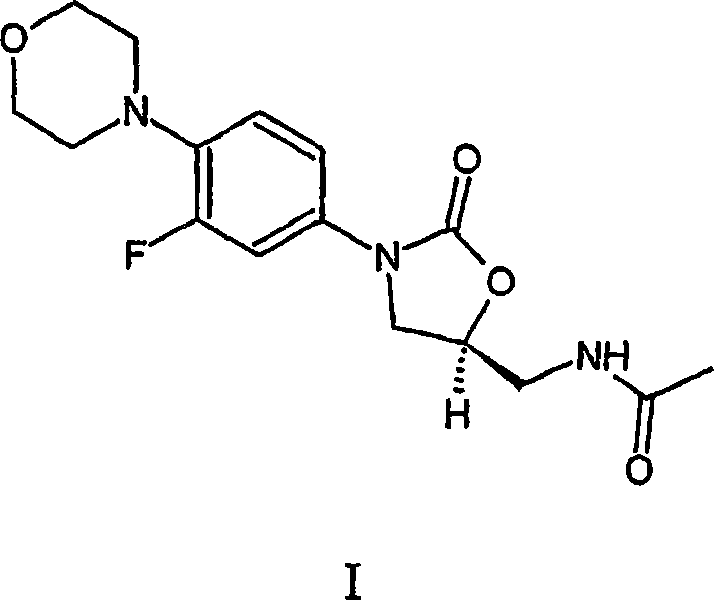
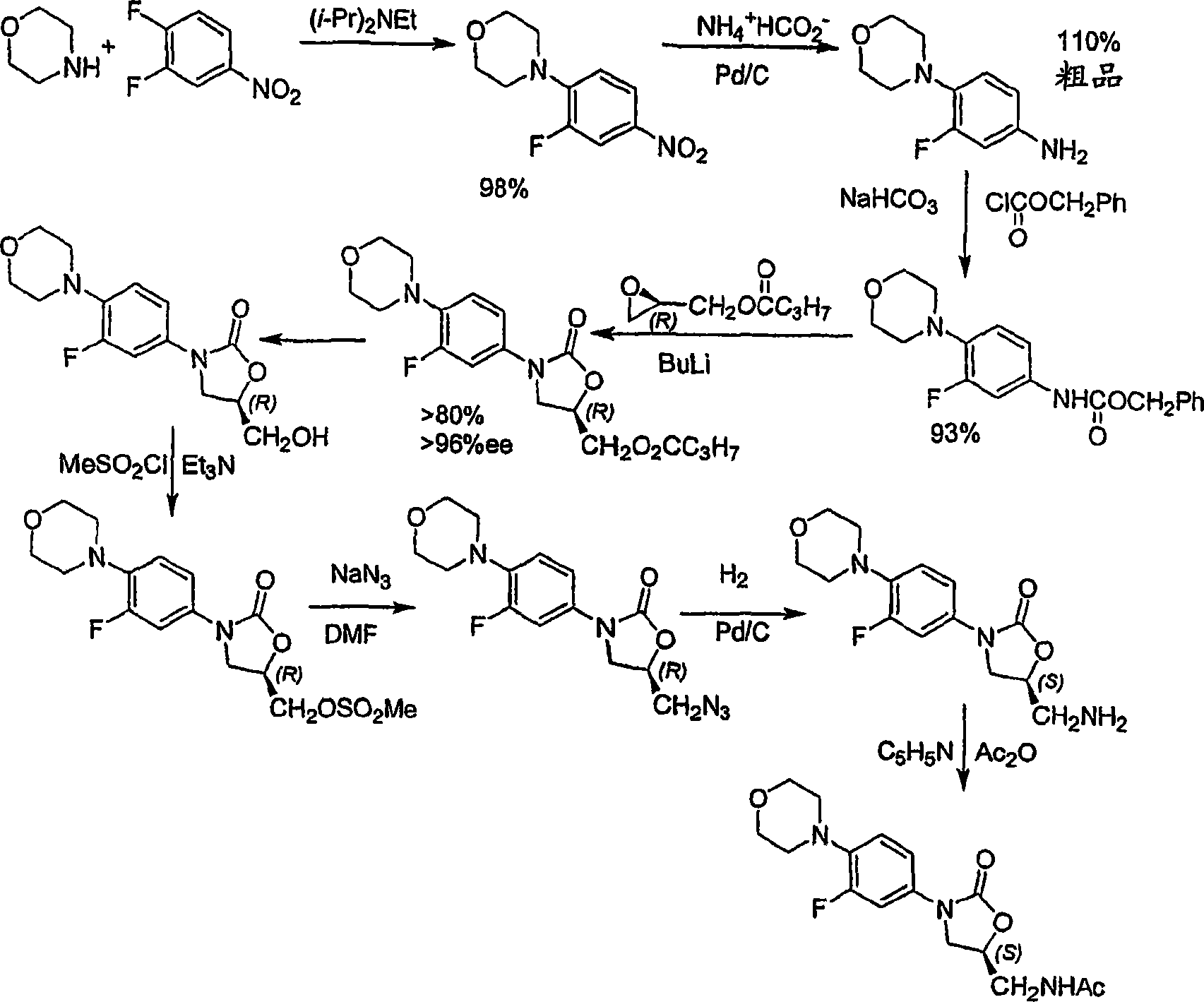
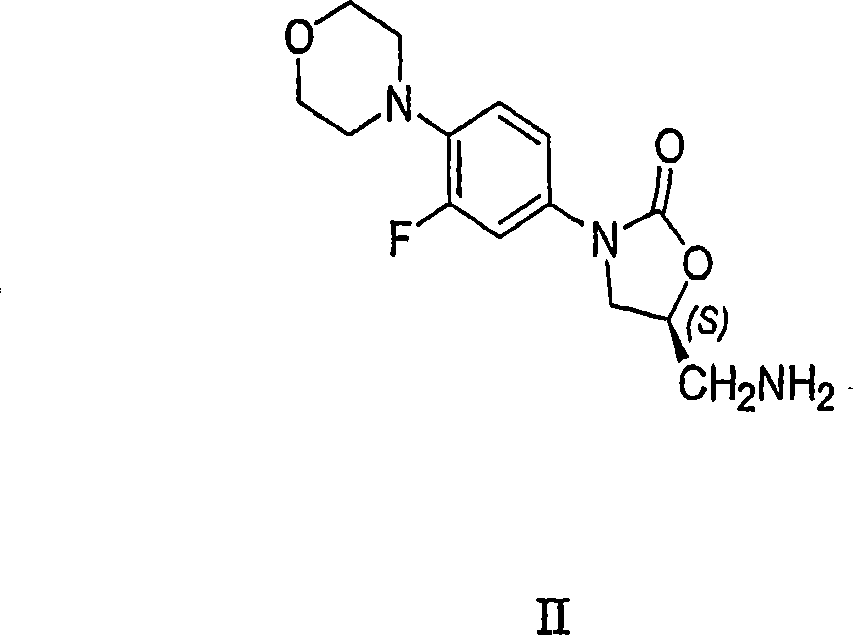
Processes for the preparation of linezolid intermediate
A technology of linezolid and pure linezolid, applied in the field of preparation of linezolid intermediates, capable of solving the problems of low yield of linezolid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] A process for the preparation of linezolid disclosed in Example 5 of the '792 patent, wherein the corresponding azide (III) is reduced to the corresponding amine (II) by hydrogenation using ethyl acetate as a solvent. In contrast, the hydrogenation in the reduction method disclosed in the present invention is carried out in the absence of ethyl acetate solvent, or in various solvents or solvent systems using ammonium formate as a reducing agent.
[0047] In one embodiment of the invention, the reduction process is carried out by catalytic hydrogenation, said method comprising:
[0048] (a) R-N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) is selected from the following except ethyl acetate Organic solvent mix: C 1 -C 8 Straight or branched fatty alcohols, C 6 -C 12 Aromatic hydrocarbons, and mono-, di-, tri-C 1 -C 4 Alkyl-substituted or unsubstituted benzenes, C other than ethyl acetate 1 -C 4 Alkyl esters and chlorinated aromatic hydroc...
Embodiment
[0088] Example 1 - Comparative Example, according to U.S. Patent No. 5,688,792
[0089] Preparation of linezolid from an azide (III) intermediate by catalytic hydrogenation
[0090] Add 6g R-N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) and 150ml ethyl acetate in 1L reactor, then Add 0.6g Pd / C. The system was flushed 3 times with nitrogen and 3 times with hydrogen. The pressure of the hydrogen gas was set to 1.5 atm. The reaction mixture was stirred at RT and monitored by periodic TLC or HPLC until complete. The reaction mixture was filtered through celite and the resulting solution was treated with acetic anhydride at RT in the presence of triethylamine. The precipitate was filtered off and dried to obtain crystalline form IV of linezolid (I) containing 3.2% of bis-linezolid (IV).
Embodiment 2
[0091] Example 2 - Preparation of Linezolid from an Azide (III) Intermediate by Catalytic Hydrogenation
[0092] Add 9g R-N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) and 150ml toluene in 1L reactor, then add 0.6 g Pd / C and 20ml ammonium hydroxide. The system was flushed 3 times with nitrogen and 3 times with hydrogen. The pressure of the hydrogen gas was set to 1.5 atm. The reaction mixture was stirred at RT and monitored by periodic TLC or HPLC until complete. The reaction mixture was filtered through celite and the resulting solution was treated with 1.5-5 equiv of acetic anhydride at RT. The formed precipitate was filtered off and dried to give Linezolid (I). No trace of bis-linezolid (4) was detected, indicating that no more than 0.01% (w / w) of bis-linezolid (4) was present.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com