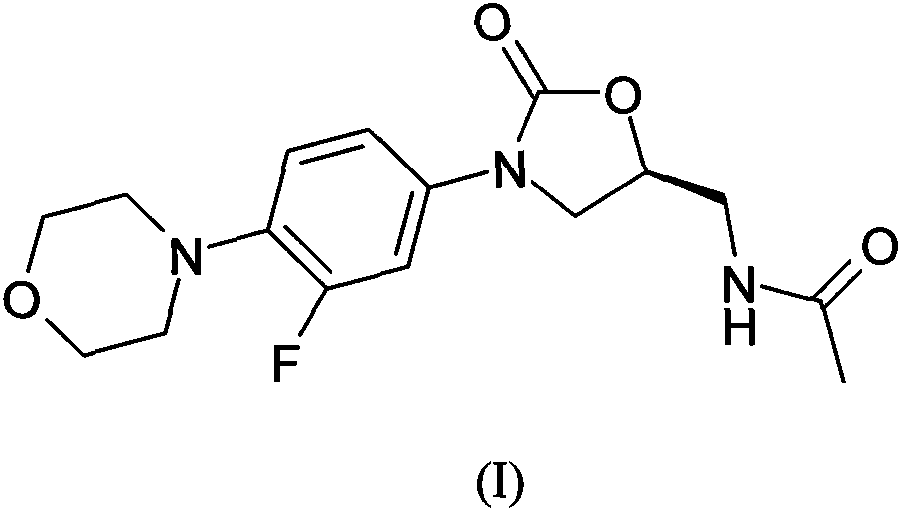
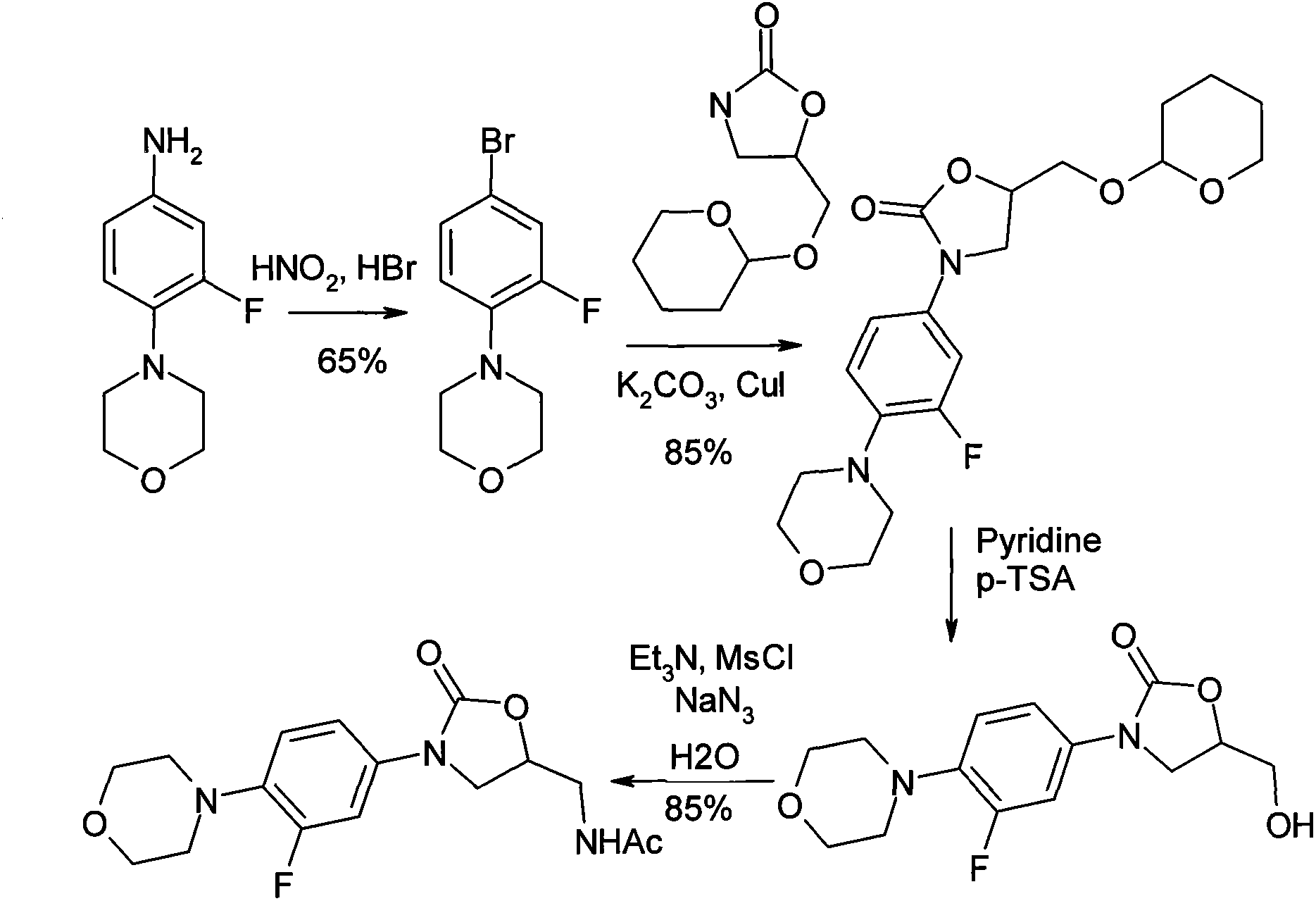
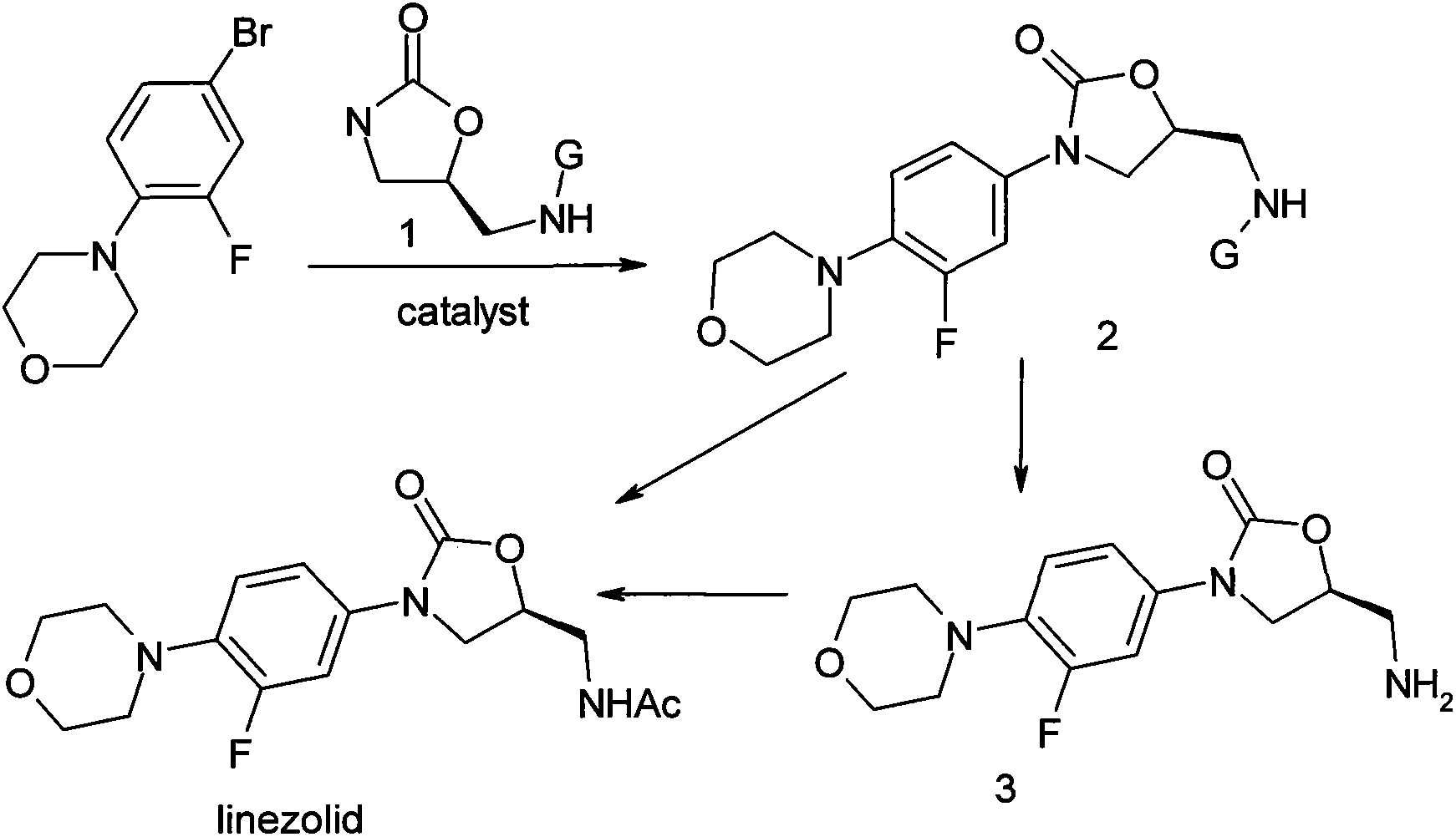
Method for preparing linezolid
A technology of linezolid and compounds, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The purified CuI (0.22mmol, 42mg), anhydrous K 2 CO 3 (7.35mmol, 1.02g), 3-fluoro-4-morpholinyl bromobenzene (4.41mmol, 1.15g) and acetyloxazolidinone (4.41mmol, 0.70g) were put into a 50mL two-mouth round bottom with a condenser In the flask. Vacuum and flush the bottle three times with nitrogen, and protect with nitrogen. A solution of (±)-1,2-cyclohexanediamine (0.22 mmol, 25 mg) in 1,4-dioxane (10 mL) was added via a syringe, and then stirred at 110°C for 20 hours. The reaction was cooled to room temperature and filtered through celite, then washed with dichloromethane (2×50 mL). The organic layers were combined, and the residue was separated and purified by silica gel column after concentration. The eluent is ethyl acetate: petroleum ether (60-90℃) (2:1) to obtain (S)-N-[[3-(3-fluoro-4-morpholinyl)phenyl]-2- Oxo-5-oxazolidinyl]methyl]acetamide 1.31 g (88%).
Embodiment 2
[0024] The purified CuI (0.22mmol, 42mg), anhydrous K 2 CO 3 (7.35mmol, 1.02g), 3-fluoro-4-morpholinyl bromobenzene (4.41mmol, 1.15g) and oxazolidinone (4.41mmol, 0.51g) were put into a 50mL two-necked round bottom flask with a condenser in. Vacuum and flush the bottle three times with nitrogen, and protect with nitrogen. A solution of (±)-1,2-cyclohexanediamine (0.22 mmol, 25 mg) in 1,4-dioxane (10 mL) was added via a syringe, and then stirred at 110°C for 20 hours. The reaction was cooled to room temperature and filtered through celite, then washed with dichloromethane (2×50 mL). The organic layers were combined, and the residue was separated and purified by silica gel column after concentration. The eluent is ethyl acetate: petroleum ether (60-90℃) (1:4) to obtain (S)-N-[3-(3-fluoro-4-morpholinyl)phenyl]-2-oxy Sulfo-5-oxazolidinyl]methylamine 0.81 g (62%).
Embodiment 3
[0026] The purified CuI (0.22mmol, 42mg), anhydrous K 2 CO 3 (7.35mmol, 1.02g), 3-fluoro-4-morpholinyl bromobenzene (4.41mmol, 1.15g) and benzyloxazolidinone (4.41mmol, 0.91g) were put into a 50mL two-mouth circle with a condenser Bottom flask. Vacuum and flush the bottle three times with nitrogen, and protect with nitrogen. A solution of (±)-1,2-cyclohexanediamine (0.22 mmol, 25 mg) in 1,4-dioxane (10 mL) was added via a syringe, and then stirred at 110°C for 20 hours. The reaction was cooled to room temperature and filtered through celite, then washed with dichloromethane (2×50 mL). The organic layers were combined, and the residue was separated and purified by silica gel column after concentration. The eluent is ethyl acetate: petroleum ether (60-90℃) (3:1) to obtain (S)-N-[3-(3-fluoro-4-morpholinyl)phenyl]-2-oxo (5-oxazolidinyl]methylamine 1.10g (65%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com