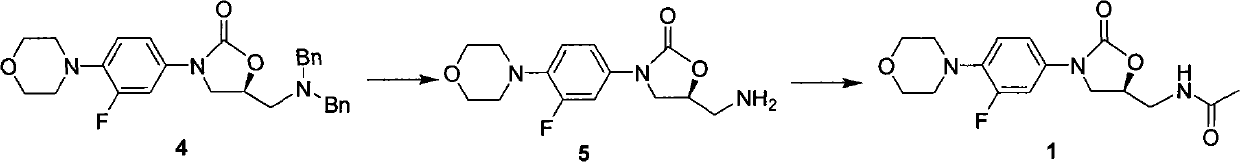
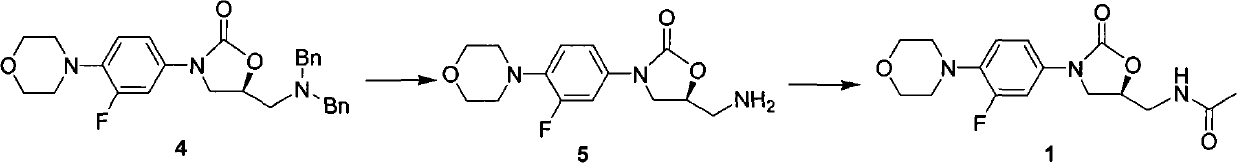
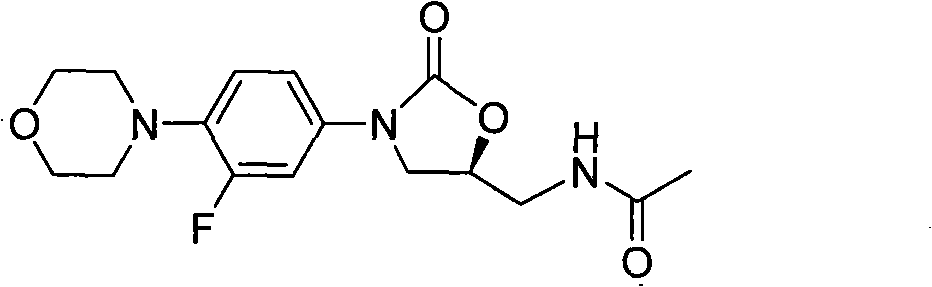
Preparation method of linezolid and intermediate thereof
一种利奈唑胺、时间的技术,应用在利奈唑胺的制备方法及其中间体领域,能够解决设备要求高、繁琐、操作条件苛刻等问题,达到后处理简单、工艺简单、易得价格的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0058] Preparation of embodiment 13-fluoro-4-morpholinyl nitrobenzene
[0059] Morpholine (60g, 0.69mol) was added in 300mL ethyl acetate, triethylamine (70g, 0.69mol) was added, 3,4-difluoronitrobenzene (100g, 0.63mol) was added dropwise, and the temperature of the feed liquid was controlled to be lower than 50°C, drop it within 1 hour. Maintain the temperature at 45-50°C for 10 hours until the reaction is complete. Extracted with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and dried to obtain 138 g of a yellow solid with a yield of 97%.
[0060] 1 H NMR (300MHz, CDCl 3 )δ: 3.28 (m, 4H, CH 2 CH 2 N), 3.88 (m, 4H, CH 2 CH 2 O), 6.92(t, 1H, Ar H ), 7.91 (dd, 1H, Ar H ), 7.99 (dd, 1H, Ar H ) HPLC: 99.1%.
Embodiment 23
[0061] Preparation of embodiment 23-fluoro-4-morpholinylaniline
[0062] Add 3-fluoro-4-morpholinylnitrobenzene (40g, 177mmol), ammonium formate (50g, 793mmol), 10% Pd / C 4.0g into 200mL ethyl acetate, heat to 45-50°C for 8h, until The response is complete. Filter, add water to separate layers, wash the organic layer with saturated brine, dry over anhydrous magnesium sulfate, filter, and distill off the solvent to obtain 33 g of solids, with a yield of 95%.
[0063] 1 H NMR (300MHz, CDCl 3 )δ: 3.01 (m, 4H, CH 2 CH 2 N), 3.56 (br, 2H, Ar NH 2 3.86 (m, 4H, CH 2 CH 2 O), 6.41 (m, 2H, Ar H ), 6.79 (m, 1H, Ar H ) HPLC: 99.0%.
Embodiment 3
[0064] The preparation of embodiment 3N-benzyloxycarbonyl-3-fluoro-4-morpholinoaniline (compound 3)
[0065] 3-Fluoro-4-morpholinoaniline (26g, 133mmol) was added to 200mL of acetone, sodium bicarbonate (17g, 202mmol) and 150mL of water were added. The feed solution was cooled to -10~0°C, benzyl chloroformate (26 g, 152 mmol) was added dropwise, and the dropwise was completed in 1 hour. The feed solution naturally rose to room temperature (25° C.), and the reaction was maintained for 2 hours. The feed solution was poured into 500 mL of ice water, filtered, and dried to obtain 39 g of off-white solid with a yield of 90%.
[0066] 1 H NMR (300MHz, CDCl 3 )δ: 3.01 (m, 4H, CH 2 CH 2 N), Ar NH 2 3.85 (m, 4H, CH 2 CH 2 O), 5.14(s, 2H, Ar CH 2 )6.93 (m, 3H, Ar H ), 7.35 (m, 6H, Ar H 、CN H c)
[0067] HPLC: 98.2%, recrystallized from ethyl acetate to obtain 37.6 g of white solid, HPLC: 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com