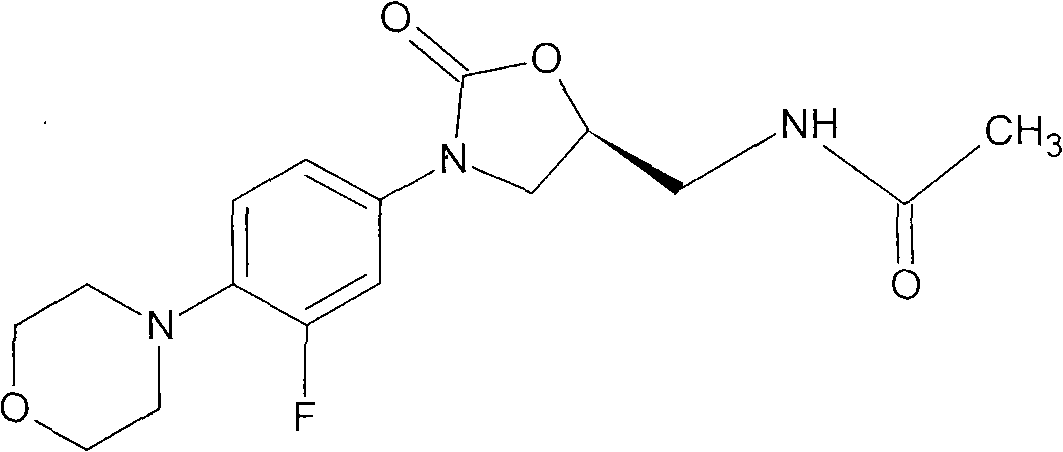
New crystal form of linezolid and preparation method and application thereof
A crystal form and crystallization technology, applied in the field of new drug crystal forms, can solve problems such as poor stability, and achieve the effects of reduced storage cost, improved stability and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of Linezolid Form V of the present invention
[0030] Take the pure product of linezolid, wherein the S enantiomer has a purity greater than 99.5%, dissolve it in ethyl acetate, add petroleum ether under stirring, and stir for 2-3 hours after the crystals are precipitated, and reduce Dry under pressure (20-30 mmHg) for about 7 hours to obtain off-white crystals, that is, linezolid type V crystals. Melting point: 175.2°C.
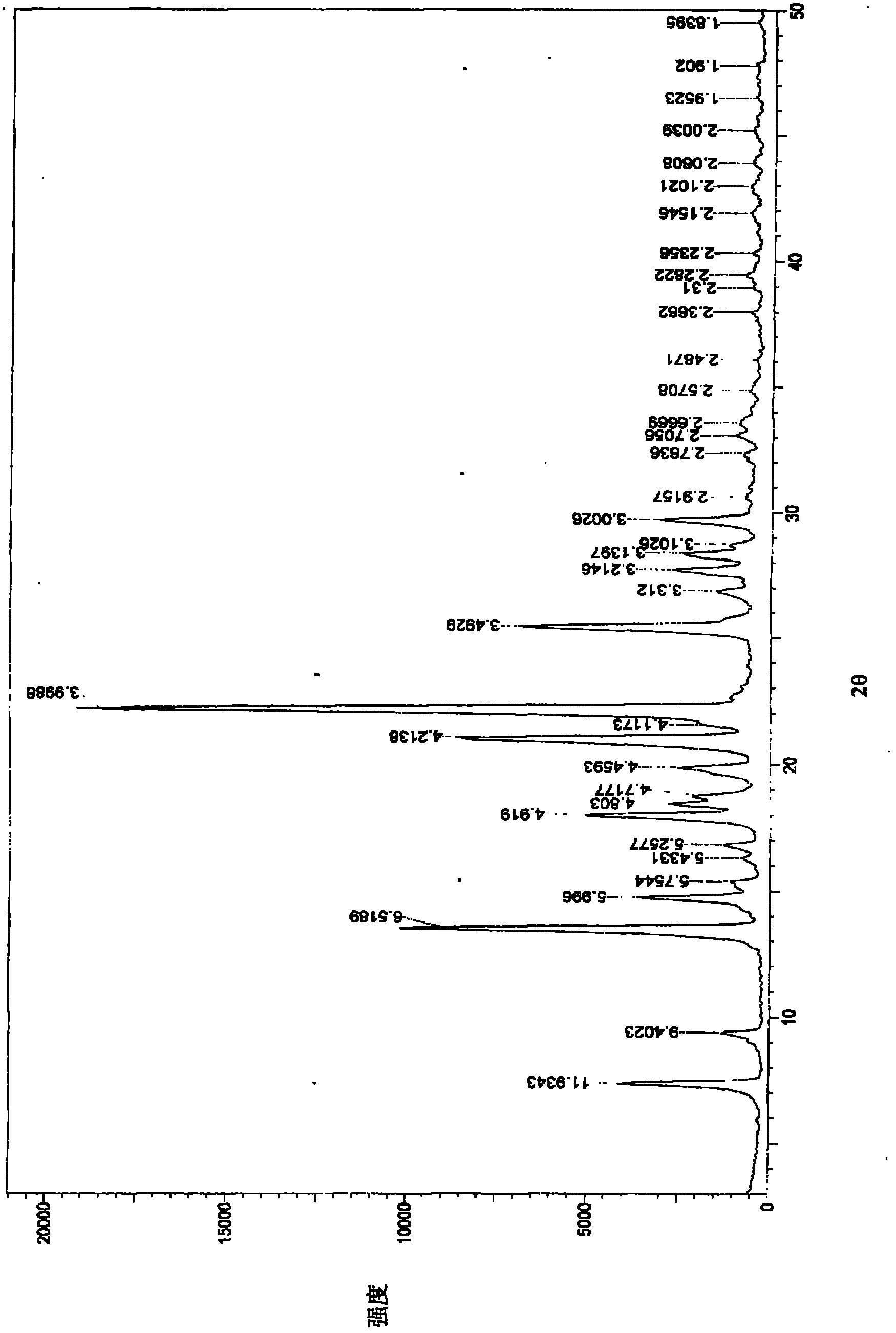
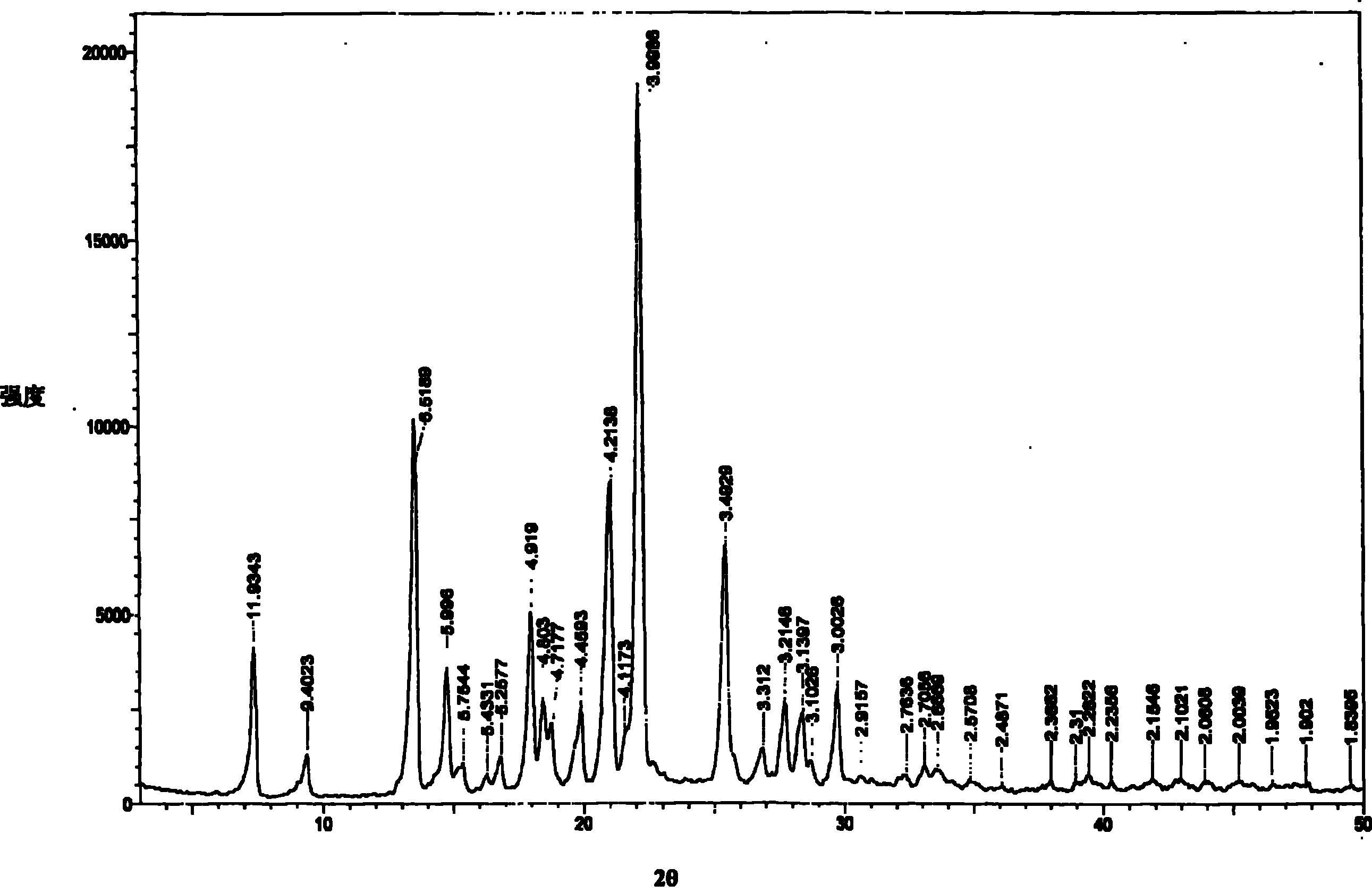
[0031] Powder X-ray diffraction measurement conditions: CuKα line, (monochromator), tube voltage 40KV, tube current 25mA. The powder X-ray diffraction measurement result of gained crystal is shown in figure 1 middle. With regard to the obtained crystal, in the diffraction pattern of powder X-ray diffraction, there are characteristic absorption peaks.
[0032] The raw material of linezolid used can be synthesized by a known method or purchased from a commercial product. For linezolid prepared by the method disclosed in US ...
Embodiment 2
[0034] Example 2 Stability investigation test of linezolid crystal form V of the present invention
[0035] To investigate the stability of this product to heat, the raw material sample is placed in a desiccator with a relative humidity of 75%, and then placed in a 40°C constant temperature drying oven. After 6 months, the sample is taken. The content determination by HPLC method: 99.69%. In the powder X-ray diffraction diagram of type V crystal, in the diffraction angle (2θ) 7.39° (relative intensity 6.99), 13.49° (relative intensity 35.34), 14.75° (relative intensity 12.55), 17.98° (relative intensity 29.79), 18.46 ° (relative intensity 19.63), 18.68° (relative intensity 12.98), 19.91° (relative intensity), 21.12° (relative intensity 62.93), 22.21° (relative intensity 100.00), 25.45° (relative intensity 28.02), 27.68° (relative Intensity 8.6), 28.35° (relative intensity 7.62), 29.72 (relative intensity 8.88) ± 0.2° have characteristic absorption peaks.
[0036] The powder X...
Embodiment 3
[0037] The powder of the new crystal form of linezolid was taken, and the injection preparation process was used to make it into a water injection injection, and the filling volume of each preparation package was 200 mg / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com