Linezolid oral suspension and preparation method theroef
An oral suspension, linezolid technology, applied in the directions of liquid delivery, pharmaceutical formulation, emulsion delivery, etc., can solve the inconvenience of clinical medication, the difficulty of dividing linezolid into doses, and the inconvenience of divided doses or divided doses of oral solid preparations. Accuracy and other issues to achieve the effect of good physical and chemical compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
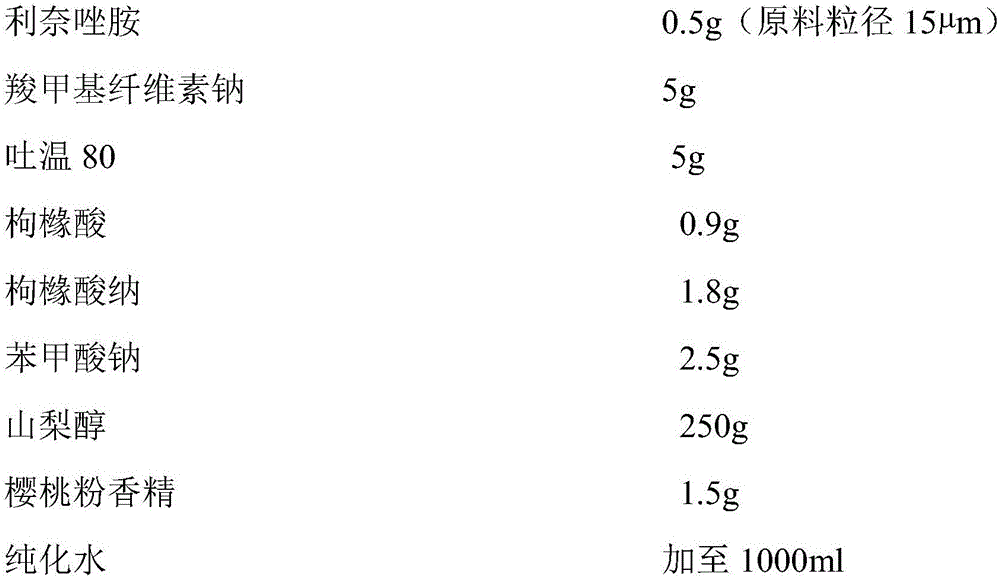
[0068] Example 1 Linezolid oral suspension formula 1 and preparation method
[0069] (1) Preparation prescription: (total made into 1000ml)
[0070]
[0071] (2) Preparation method:
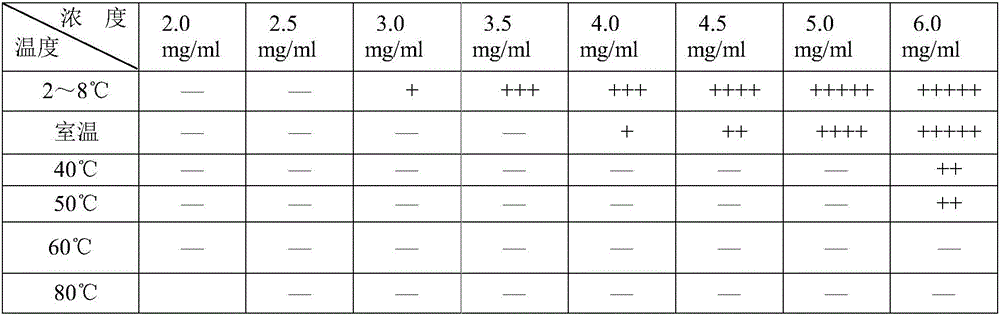
[0072] 1) Weigh 4 / 5 of the prescription amount of purified water, add the prescription amount of suspending agent sodium carboxymethyl cellulose to it, heat to 80-100°C, stir to swell and clarify, and cool to room temperature to obtain solution I;
[0073] 2) Add the wetting agent polysorbate 80 to solution I, stir and dissolve it to obtain solution II;
[0074] 3) Add linezolid micropowder to solution II, homogenize at 5000 rpm and disperse for 5 minutes, mix well, and flocculate to obtain suspension III;
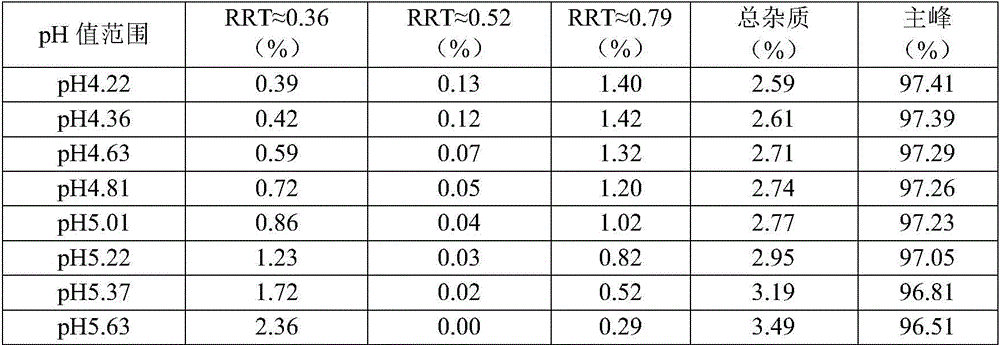
[0075] 4) Adding the pH regulators sodium citrate and citric acid to suspension Ⅲ to obtain suspension Ⅳ;
[0076] 5) Finally, add the prescription amount of preservative sodium benzoate, sweetener sorbitol and corrective cherry powder flavor to suspension IV, stir at 30Hz for 10min, after dissolving, ad...
Embodiment 2
[0077] Example 2 Linezolid oral suspension formulation 2 and preparation method
[0078] (1) Preparation prescription: (total made into 1000ml)
[0079]
[0080] (2) Preparation method:
[0081] 1) Weigh 4 / 5 of the prescription amount of purified water, add the prescription amount of suspending agent sodium carboxymethyl cellulose to it, heat to 80-100°C, stir to swell and clarify, and cool to room temperature to obtain solution I;
[0082] 2) Add the wetting agent polysorbate 80 to solution I, stir and dissolve it to obtain solution II;
[0083] 3) Add linezolid micropowder to solution II, homogenize at 5000 rpm and disperse for 8 minutes, mix and flocculate to obtain suspension III;
[0084] 4) Adding the pH regulators sodium citrate and citric acid to suspension Ⅲ to obtain suspension Ⅳ;
[0085] 5) Finally, add the prescription amount of preservative sodium benzoate, sweetener sorbitol and corrective cherry powder flavor to suspension IV, stir at 30Hz for 10min, after dissolving, add ...
Embodiment 3
[0086] Example 3 Linezolid oral suspension formulation 3 and preparation method
[0087] (1) Preparation prescription: (total made into 1000ml)
[0088]
[0089]
[0090] (2) Preparation method:
[0091] 1) Weigh 4 / 5 of the prescription amount of purified water, add the prescription amount of suspending agent sodium carboxymethyl cellulose to it, heat to 80-100°C, stir to swell and clarify, and cool to room temperature to obtain solution I;
[0092] 2) Add the wetting agent polysorbate 80 to solution I, stir and dissolve it to obtain solution II;
[0093] 3) Add linezolid micropowder to solution II, homogenize at 5000 rpm and disperse for 8 minutes, mix and flocculate to obtain suspension III;
[0094] 4) Adding the pH regulators sodium citrate and citric acid to suspension Ⅲ to obtain suspension Ⅳ;
[0095] 5) Finally, add the prescription amount of preservative sodium benzoate, sweetener sorbitol and corrective cherry powder flavor to suspension IV, stir at 30Hz for 10min, after dissolv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com