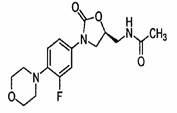
Preparation method for antibacterial drug linezolid
A technology of linezolid and acetamide, applied in the field of organic compound preparation, can solve the problems of high cost of industrialized production, difficult to obtain raw materials, complicated process and the like, and achieve the effects of safe industrialized production, convenient industrialized production and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation method of linezolid comprises the following steps: Linezolid is purified after condensation reaction in a solvent, the condensing agent is lithium tert-butoxide, the solvent is a mixed solvent of dimethylformamide, methanol and dichloromethane, dimethylformamide, methanol and dichloromethane The volume ratio of methyl chloride is: 7-9:0.8-1.2:75-85.
[0022] The condensation reaction is: add N-benzyloxyhydroxy-3-fluoro-4-morpholinoaniline, dimethylformamide, methanol, dichloromethane and lithium tert-butoxide into a container, stir, and add (S) -N-[2-acetoxy-3-chloropropyl]acetamide, heat preservation reaction at 35-45°C for 8-14 hours.
[0023] The purification is as follows: adding saturated NH4Cl, water, and saturated saline in sequence, standing for stratification, separating the organic layer, and distilling off the solvent to obtain an oily substance, which is refined with ethyl acetate and dried to obtain a white linezolid solid ; mp178-182°C, y...
Embodiment 2
[0025] 1. Synthesis of 3-fluoro-4-morpholinonitrobenzene
[0026] In a 500 ml three-necked flask, add 18 g of morpholine, 150 ml of ethyl acetate, and 20.4 g of triethylamine. While stirring at room temperature, slowly add 32.15 g of 3,4-difluoronitrobenzene dropwise at room temperature (25°C) Stir overnight, add 250 ml of ethyl acetate for extraction, wash with 250 ml of saturated brine, let stand to separate the layers, separate the organic layer, evaporate the solvent, and dry at 50°C to obtain 44.3 g of yellow solid, yield 95.3%, mp 108 °C-109 °C.
[0027] 2. Synthesis of N-benzyloxyhydroxy-3-fluoro-4-morpholinoaniline
[0028] In a 500 ml three-necked flask, add 27.2 g of 3-fluoro-4-morpholino nitrobenzene, 2.6 g of 10% Pd / C, 28.7 g of ammonium formate, and 360 ml of acetone, stir, and at an internal temperature of 47°C React for 3 h, cool to room temperature, filter with suction, transfer the filtrate to a 1000 ml three-necked flask, add 360 ml acetone and 22.4 g NaH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com