Crystalline forms of linezolid intermediate
a linezolid intermediate and crystalline form technology, applied in the field of solid state chemistry of the linezolid intermediate, can solve the problem of producing an undesirable level of reaction by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
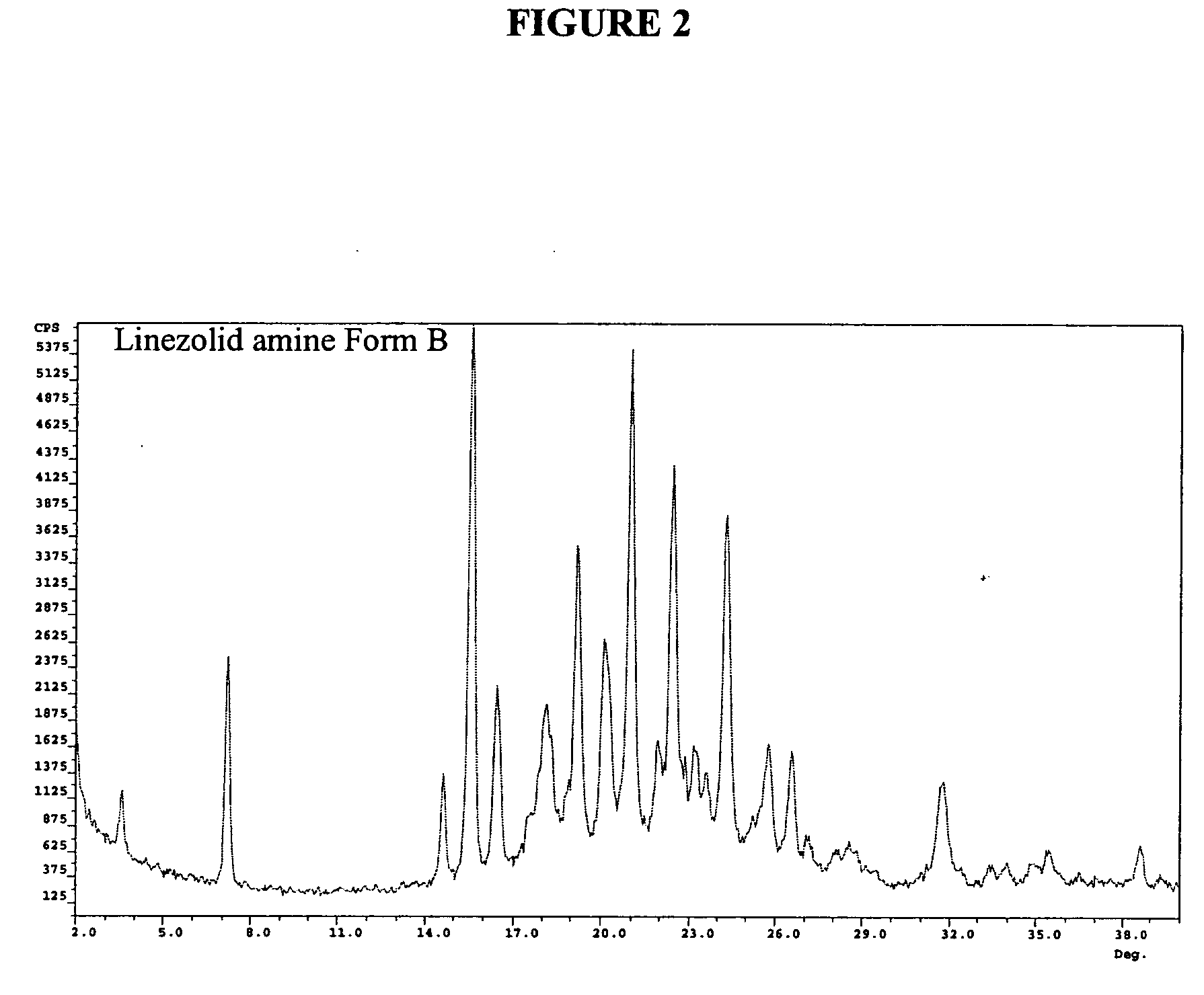
Preparation of Intermediate Amine (II) Crystalline Form B
[0034] In a 1L reactor, 6 g R—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) was charged with 150 ml ethyl acetate, followed by 0.6 g 10% Pd / C. The system was flushed 3 times with nitrogen and 3 times with hydrogen. The pressure of hydrogen was set to 1.5 atm. The reaction mixture was stirred at RT and the reaction followed by TLC or HPLC until completion. The reaction mixture was filtered through celite and the solution was evaporated to obtain 4.6 g S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) as a white solid. The crystals were analyzed by PXRD and showed a novel form of) the intermediate amine (II) (Form B, 91.7% total purity by HPLC).
example 2
Preparation of Intermediate Amine (II) Crystalline Form C
[0035] In a 10L reactor, 150 g R—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) was charged, followed by 15 g Pd / C in 5L toluene. Finally 500 ml ammonium hydroxide was added. The system was flushed 3 times with nitrogen and 3 times with hydrogen. The pressure of hydrogen was set to 1.5 atm. The reaction mixture was stirred at RT and the reaction followed by TLC or HPLC until completion. The reaction mixture was filtered through celite. S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) precipitated on standing and / or cooling as a white solid, was filtered, and dried at 50° C. overnight. The crystals obtained were analyzed by PXRD and showed a novel form of the intermediate amine (II) (Form C, 98.6% total purity by HPLC).
example 3
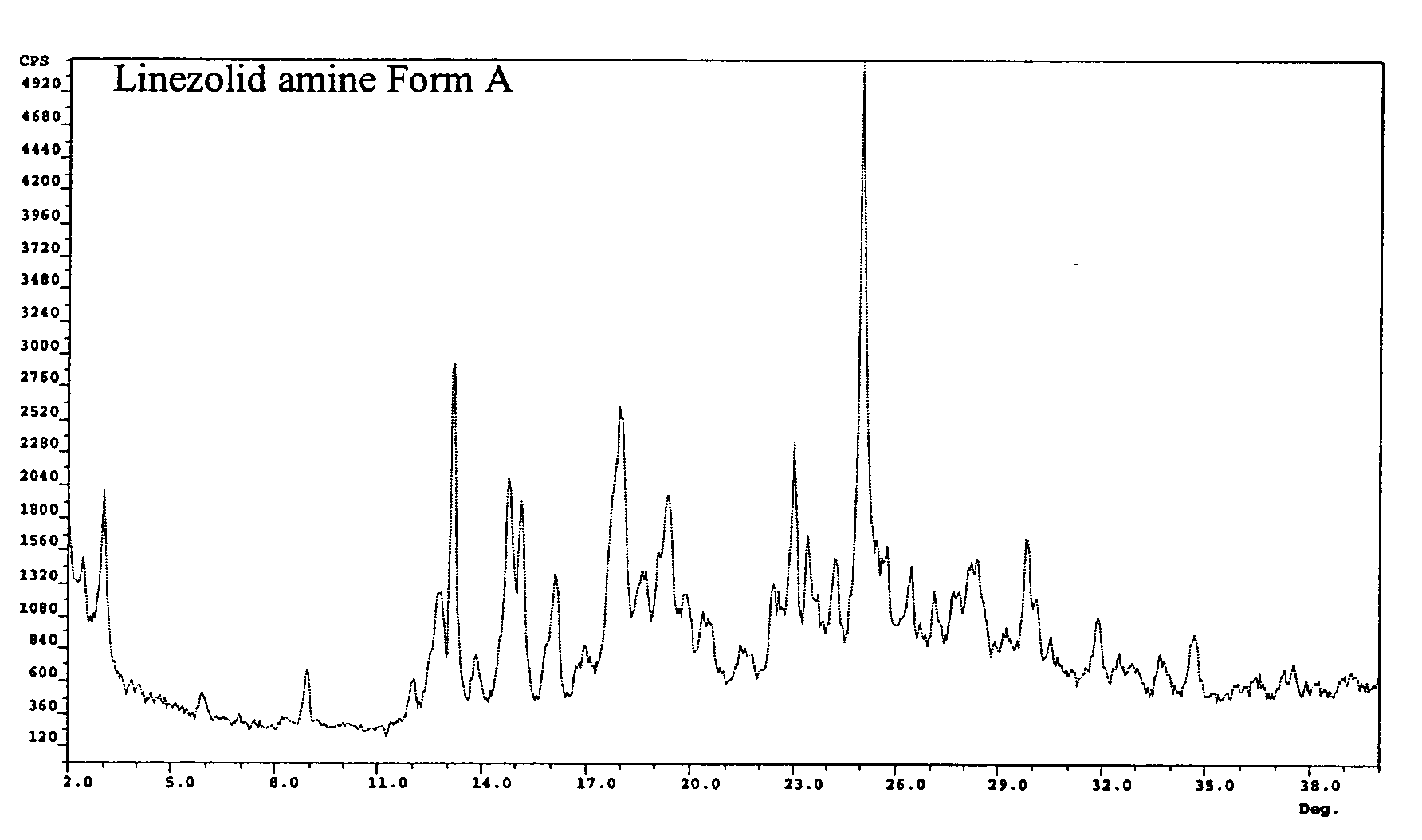
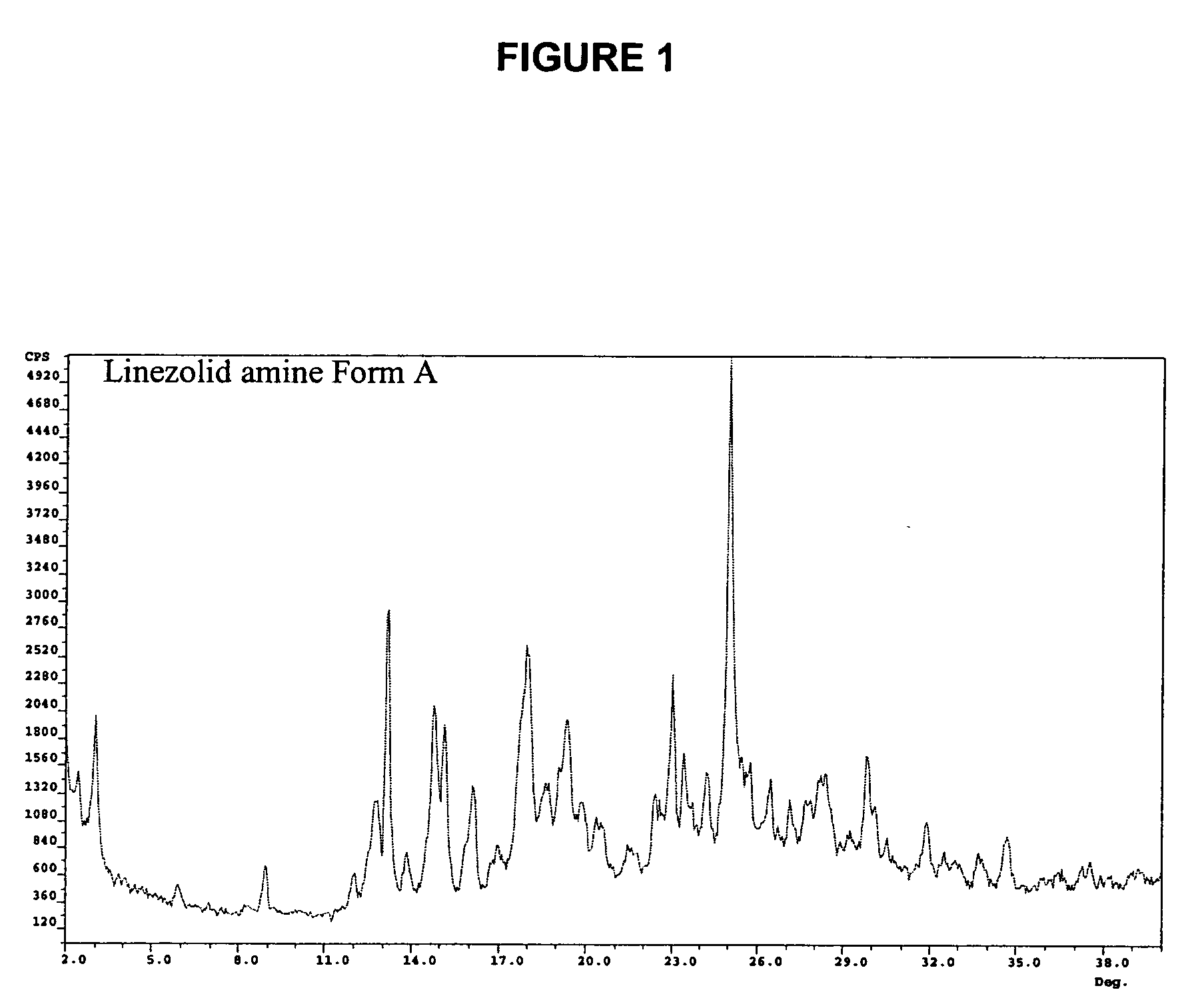
Preparation of Intermediate Amine (II) Crystalline Form A
[0036] In a three necked flask, 6.4 g R—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) was charged, followed by 2.5 g ammonium formate, 23 ml ethanol, and 2.6 g zinc powder. The reaction mixture was stirred at RT and the reaction followed by TLC or HPLC until completion. 60 ml acetone were then added. The reaction mixture was filtered and by evaporation S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) was obtained as a solid. The crystals obtained were analyzed by PXRD and showed a novel form of the intermediate amine (II) (Form A, 96.5% total purity by HPLC).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| physical properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com