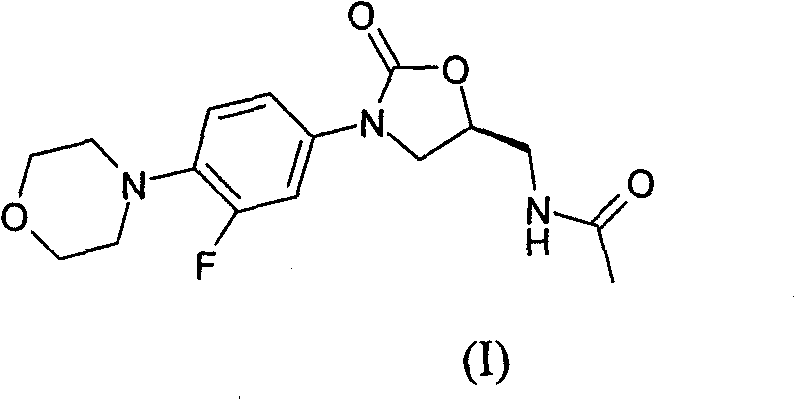
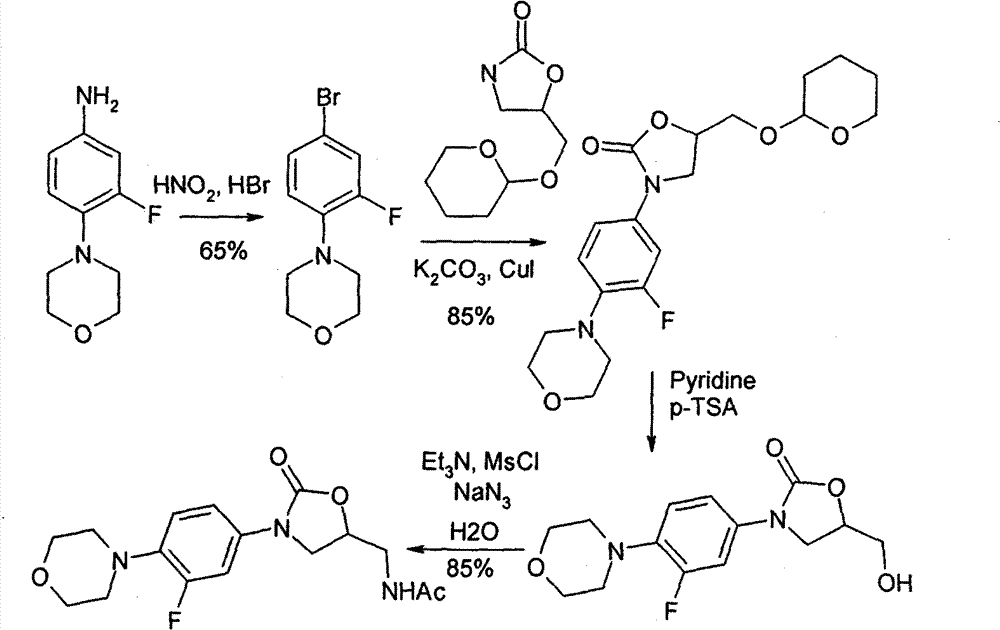
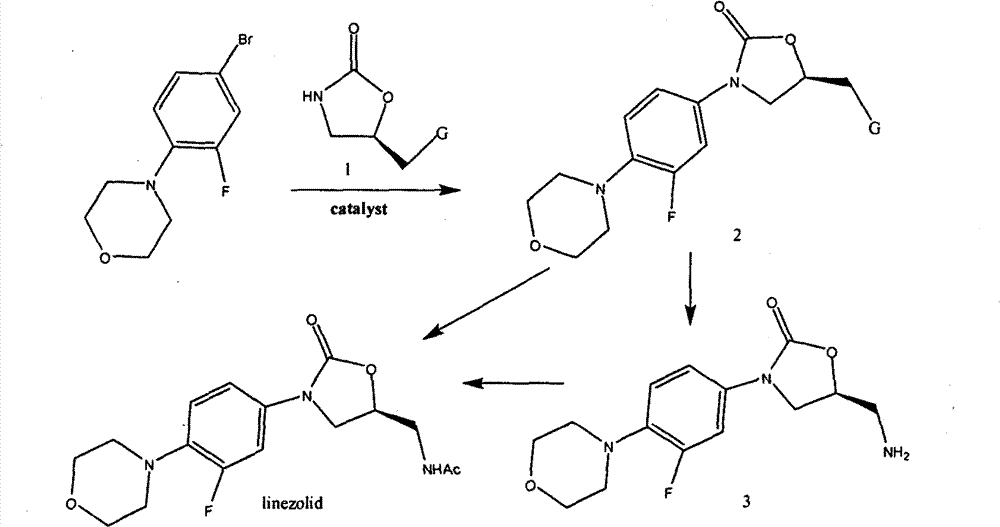
Method for preparing linezolid
A technology of linezolid and a synthesis method, applied in the field of drug synthesis, can solve problems such as danger, and achieve the effects of stable yield, high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of (S)-N-[[3-(3-fluoro-4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] from 3-fluoro-4-morpholinobromobenzene Methyl]acetamide
[0030]
[0031] Purified CuI (0.22mmol, 42mg), anhydrous K 2 CO 3 (7.35mmol, 1.02g), 3-fluoro-4-morpholino bromobenzene (4.41mmol, 1.15g) and acetyloxazolidinone (4.41mmol, 0.70g) were put into a 50mL two-port round bottom with a condenser in the flask. Evacuate and flush the bottle three times with nitrogen and blanket with nitrogen. A solution of (±)-1,2-cyclohexanediamine (0.22 mmol, 25 mg) in 1,4-dioxane (10 mL) was added via syringe, followed by stirring at 110° C. for 20 hours. The reaction was cooled to room temperature and filtered through celite, then washed with dichloromethane (2 x 50 mL). The organic layers were combined and concentrated, and the residue was separated and purified by silica gel column. The eluent is ethyl acetate:petroleum ether (60-90°C) (2:1) to obtain (S)-N-[[3-(3-fluoro-4-morpholinyl)phenyl]-2-...
Embodiment 2
[0034] Preparation of (S)-N-[3-(3-fluoro-4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]tert from 3-fluoro-4-morpholinobromobenzene butoxycarbamide methane
[0035]
[0036] Purified Cul (0.22mmol, 42mg), anhydrous K 2 CO 3 (7.35mmol, 1.02g), 3-fluoro-4-morpholinyl bromobenzene (4.41mmol, 1.15g) and Boc-oxazolidinone (4.41mmol, 0.95g) were dropped into a 50mL two-port circle with a condenser in the bottom flask. Evacuate and flush the bottle three times with nitrogen and blanket with nitrogen. A solution of (±)-1,2-cyclohexanediamine (0.22 mmol, 25 mg) in 1,4-dioxane (10 mL) was added via syringe, followed by stirring at 110° C. for 20 hours. The reaction was cooled to room temperature and filtered through celite, then washed with dichloromethane (2 x 50 mL). The organic layers were combined and concentrated, and the residue was separated and purified by silica gel column. The eluent is ethyl acetate:petroleum ether (60-90°C) (3:1) to obtain (S)-N-[3-(3-fluoro-4-morpholin...
Embodiment 3
[0039] Preparation of (S)-N-[3- (3-Fluoro-4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methylamine
[0040]
[0041](S)-N-[3-(3-fluoro-4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]benzylaminomethane (1mmol, 385mg), formic acid in methanol Solution (10mL, 4.4%) and fresh palladium carbon (10%, 50mg) were added in the hydrogenation bottle, in 5atm of hydrogen, stirred at room temperature for 15 hours, filtered to remove the catalyst, concentrated and passed through silica gel (eluent was acetic acid Ethyl ester: Petroleum ether (60-90°C) (1:4)) to separate (S)-N-[3-(3-fluoro-4-morpholinyl)phenyl]-2-oxo-5- Oxazolidinyl]methylamine 271 mg (92% yield). 1 H NMR (CDCl 3 , 400MHz) δ1.47(s, 2H), 3.05(t, J=4.5Hz, 4H), 3.87(t, J=4.5Hz, 4H), 2.95-4.03(m, 4H), 4.64-4.70(m , 1H), 6.93(t, J=9.1Hz, 1H), 7.13-7.15(dd, J 1 =8.8Hz,J 2 = 1.4Hz, 1H), 7.45-7.49 (dd, J 1 =14.5Hz,J 2 =2.4Hz, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com