Preparation method of IV crystal linezolid tablets having high drug loading capacity and capable of quickly dissolving
A linezolid tablet, high drug loading technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of not being able to achieve rapid disintegration, and achieve high loading The effect of the dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
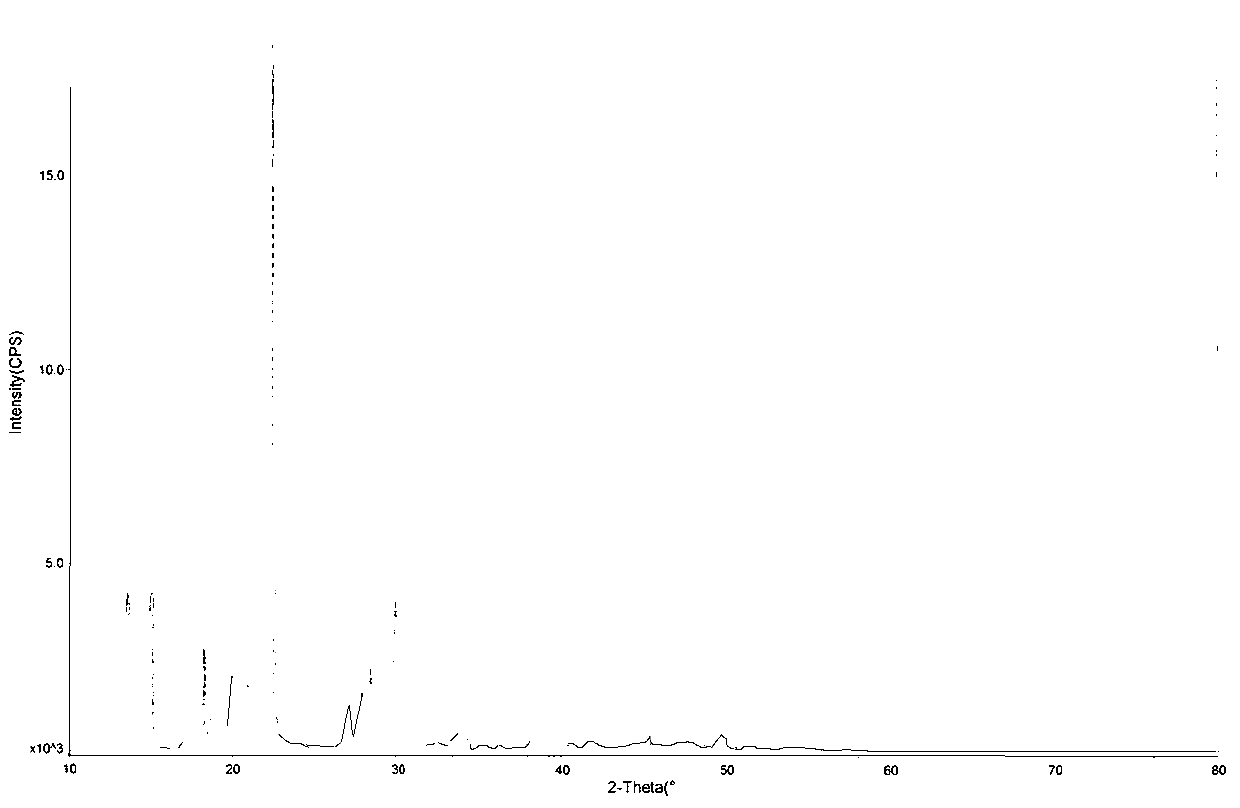
Image
Examples
Embodiment 1
[0062] Weigh the raw and auxiliary materials according to prescription 1 and implement according to the following production process:
[0063] 1. Pass linezolid through a 80-mesh sieve; corn starch, hydroxypropyl cellulose (low substitution), carboxymethyl starch sodium, microcrystalline cellulose, and povidone K30 pass through a 100-mesh sieve, and set aside.
[0064] 2. Weigh linezolid, corn starch, hydroxypropyl cellulose (low substitution), sodium carboxymethyl starch, microcrystalline cellulose, povidone K according to the prescription instructions 30 , silicon dioxide, magnesium stearate materials, spare.
[0065] 3. Put linezolid, corn starch, hydroxypropyl cellulose (low substitution), sodium starch glycolate, microcrystalline cellulose, povidone K30, and silicon dioxide in a multi-directional motion mixer, mix for 20 minutes, and collect materials.
[0066] 4. Put the mixed material in the tank mixer, start stirring, and add an appropriate amount of isopropanol sol...
Embodiment 2
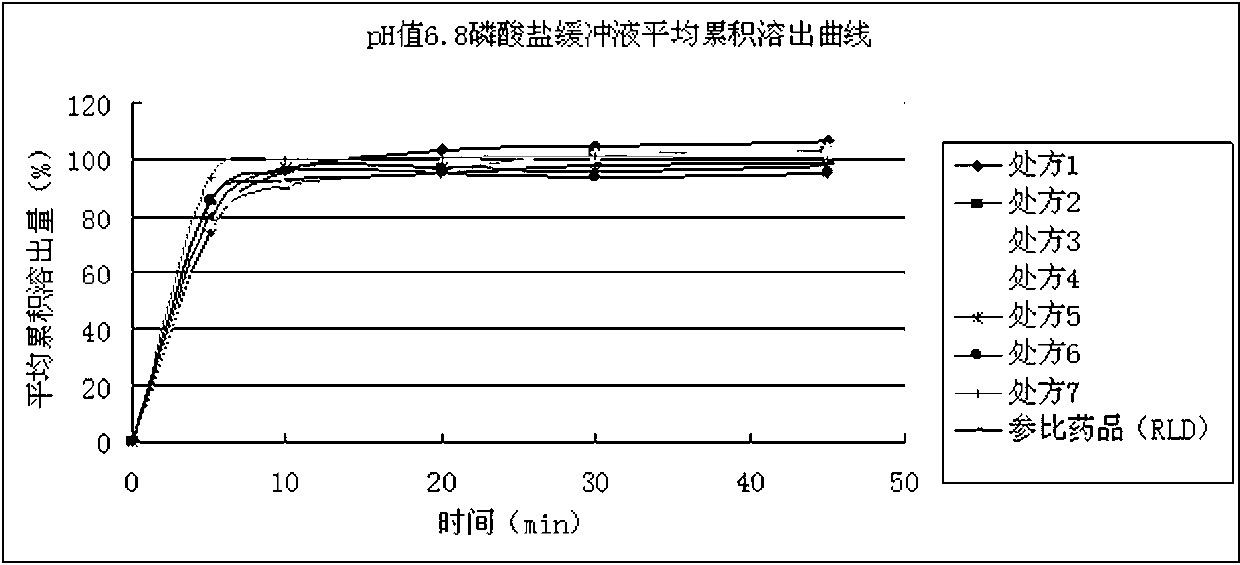
[0077] Example 2 Dissolution Determination
[0078] method :
[0079] Take this product, according to the dissolution test method (Chinese Pharmacopoeia 2010 edition two appendix Ⅹ C second method), with pH6.8 phosphate buffer (take 129.6g of potassium dihydrogen phosphate and 147.6g of dipotassium hydrogen phosphate, add water to dissolve And dilute to 2000ml, as the stock solution. Take 50ml of the stock solution and dilute to 900ml with water) as the solvent, the speed is 50 rpm, and the operation is according to the law. At 30 minutes, take 10ml of the solution, filter it, add pH 6.8 phosphate buffer to dilute to a solution containing about 10μg of linezolid per 1ml, and use it as the test solution; Determined, use pH 6.8 phosphate buffer to make a solution containing about 10 μg of linezolid per 1 ml, as a reference solution. Take the above two solutions, according to the visible-ultraviolet spectrophotometric method (Chinese Pharmacopoeia 2010 Edition, Appendix IV A)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com