Evaporation crystallization process for linezolid with crystal form I
A technology of linezolid and evaporation crystallization, which is applied in the field of compound synthesis technology, can solve problems such as difficulty in realizing industrial production, low yield, and difficult solvent recovery, and achieve low solvent usage, high yield, and fast evaporation speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
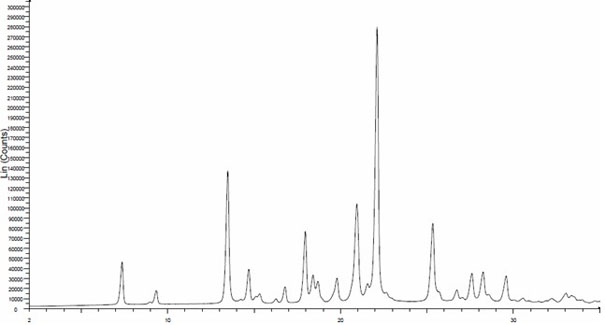
[0017] Add 100 g of crude linezolid to a four-neck flask, add 300 g of n-propanol, stir and heat to 90°C for complete dissolution, evaporate 180 g of n-propanol under reduced pressure at 80°C, crystallize by evaporation, and precipitate crystals. Lower the temperature by 10°C, add the evaporated 180g of n-propanol back into the four-neck flask, cool to 20°C, filter, and wash the filter cake with n-heptane with a mass ratio of 90g, at 40°C, -0.09Mpa Vacuum drying gave the crystalline form I linezolid with a yield of about 90.0%. The X-ray powder diffraction (XRPD) pattern of crystalline form I linezolid is shown in figure 1 .
Embodiment 2
[0019] Add 100 g of crude linezolid and 500 g of n-butanol into a four-neck flask, stir and heat to 90°C for complete dissolution, evaporate 350 g of n-butanol under reduced pressure at 95°C, crystallize by evaporation, and precipitate crystals. Cool down to 20°C, add the evaporated 350g of n-butanol back into the four-neck flask, cool to 30°C, filter, and wash the filter cake with n-heptane with a mass ratio of 170g, at 40°C, -0.09Mpa Vacuum drying gave the crystalline form I linezolid with a yield of about 85.5%.
Embodiment 3
[0021] Add 100 g of linezolid crude product and 400 g of isobutanol into a four-necked flask, stir and heat to 90°C for complete dissolution, distill 280 g of isobutanol at 100°C under reduced pressure, crystallize by evaporation, and precipitate crystals. Lower the temperature by 15°C, add the evaporated 280g of isobutanol back into the four-neck flask, cool to 30°C, filter, and wash the filter cake with n-heptane with a mass ratio of 90g, at 50°C, -0.08Mpa Vacuum drying gave the crystalline form I linezolid with a yield of about 87.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com