Non-steroid anti-inflammatory medicine for external use for ophthalmology
A non-steroidal anti-inflammatory, ophthalmic technology, applied in the direction of anti-inflammatory agents, antipyretics, drug combinations, etc., can solve problems such as inability to be used externally to the eye, and achieve the effect of less toxic side effects and strong penetrating power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
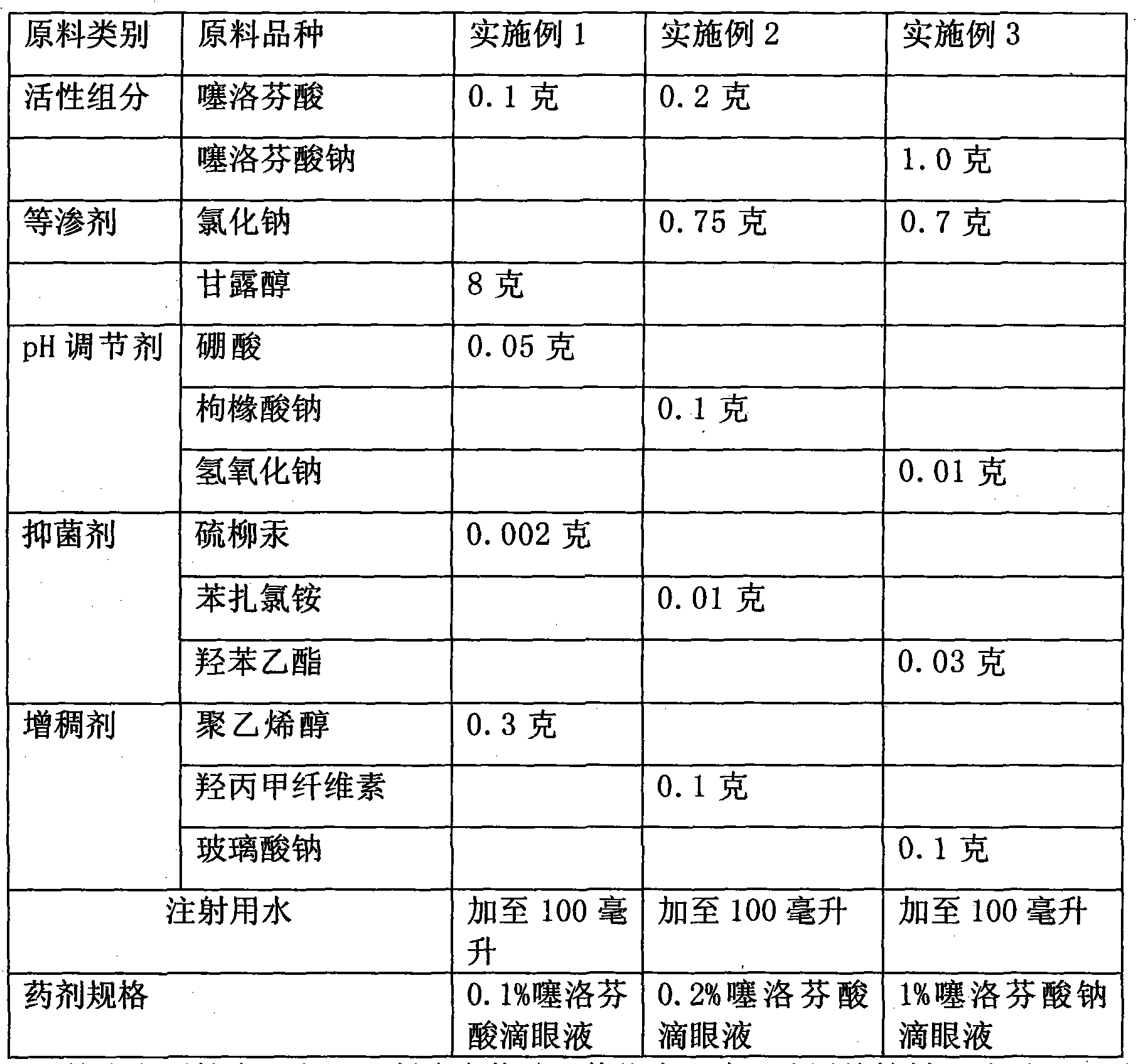
[0023] Embodiment 1-3 Preparation of tiaprofen acid and its salt eye drops raw material components and consumption
[0024]
[0025] According to the technical scheme of the present invention, the optional auxiliary material kind for preparing tiaprofen acid and its salt eye drops is not limited to the kind listed in the above table, and can also have the following multiple options:
[0026] Bacteriostatic agent: except phenylmercuric nitrate, any bacteriostatic agent known in pharmacy can be used, and its dosage is according to the conventional dosage in pharmacy. For example, ① thimerosal 0.002% to 0.005% (volume-weight percentage, that is, the number of grams per 100 ml, the same below); The effective concentration is 0.002% to 0.01%; ③alcohols, commonly used chlorobutanol 0.3~0.6%; ④parabens, commonly used ethylparaben, concentration 0.03~0.06%; The concentration is 0.01-0.08%.
[0027] Thickener: thickeners commonly used in pharmacy can be used, such as any one or al...
Embodiment 4-6
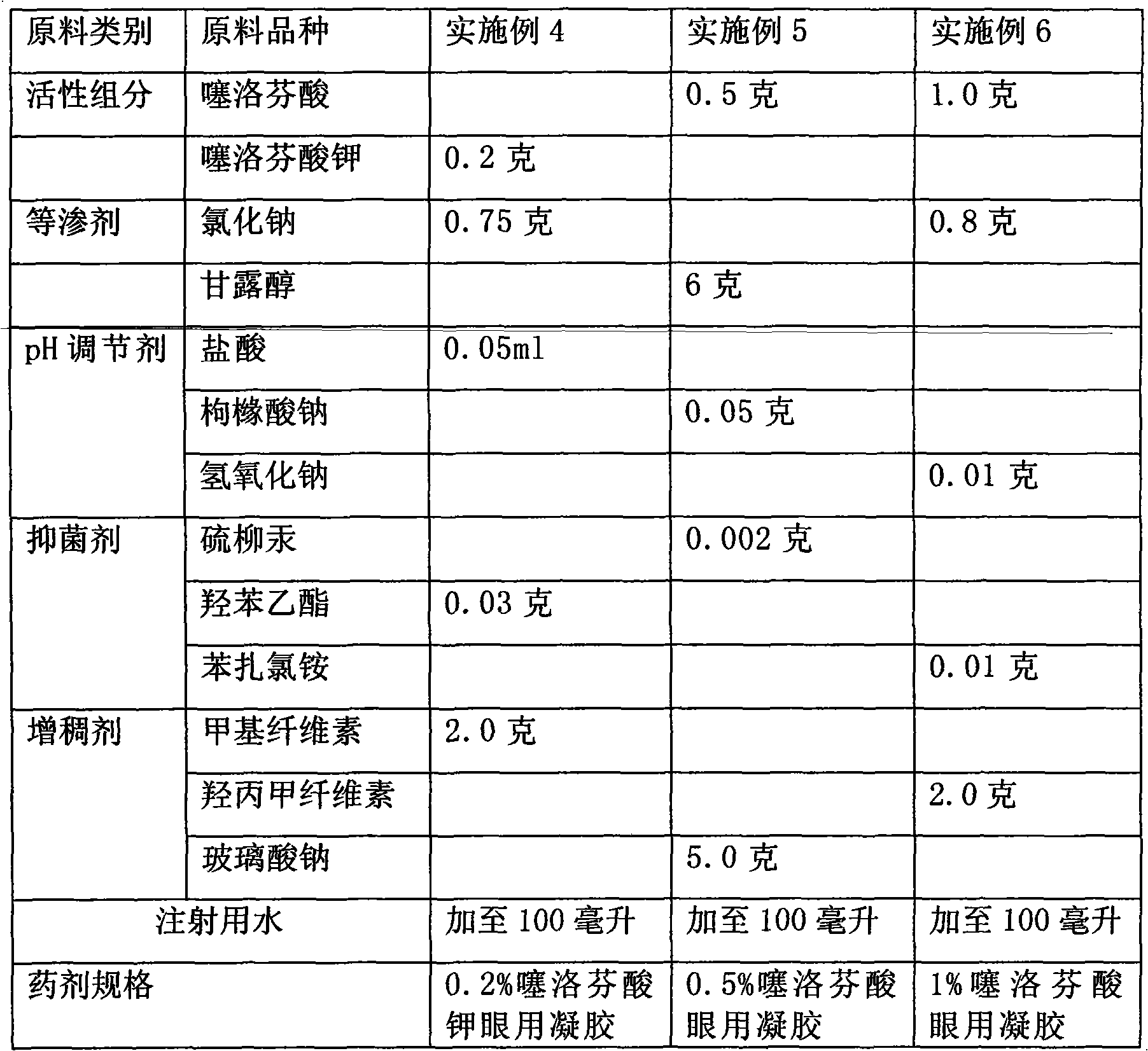
[0030] Example 4-6 Preparation of tiaprofen acid and its saline ophthalmic gel raw material components and dosage
[0031]
[0032] According to the technical scheme of the present invention, the optional auxiliary materials for preparing tiaprofen acid and its salt ophthalmic gel are not limited to the varieties listed in the above table, and can also have the following multiple options:
[0033] Wherein, the kind selection and consumption of antibacterial agent are the same as embodiment 1~3.
[0034] The thickener is selected from hypromellose, methylcellulose, sodium hyaluronate, polyvinyl alcohol, polycarbophil, polyvinylpyrrolidone, carboxymethylcellulose, carbomer, chondroitin sulfate or Any combination of the varieties; the dosage ratio of the thickener to tiaprofen acid and its salt is 0.5-5 grams: 0.1-1.0 grams.
[0035] Use a pH regulator to adjust the pH value of the finished eye drops to 5.5 to 7.5; the pH regulator is any one of sodium hydroxide, hydrochloric...
Embodiment 7-9
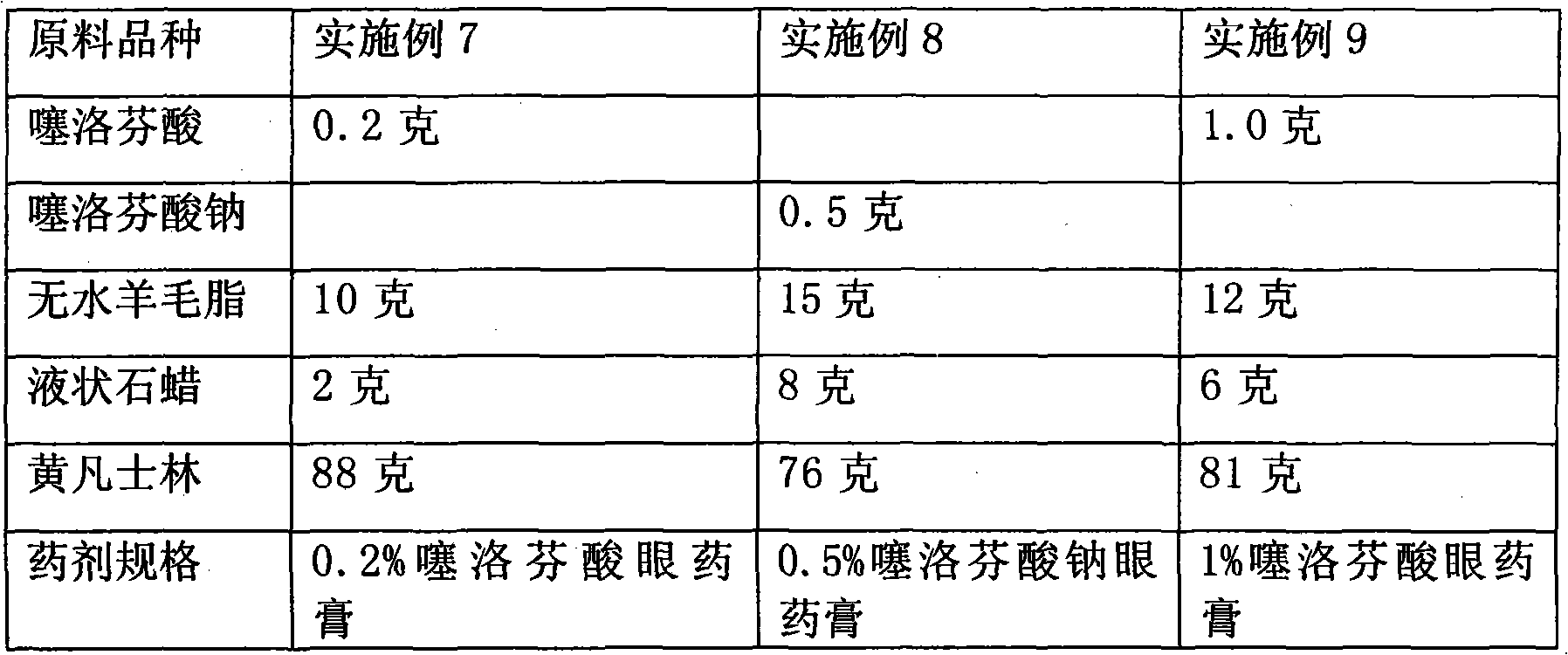
[0037] Embodiment 7-9 Preparation of tiaprofen acid and its salt eye ointment raw material components and dosage
[0038]
[0039] Preparation method: the eye ointment is prepared by the production method of eye ointment commonly used in pharmacy. Its production method is to take tiaprofen acid and its salt, add appropriate amount of sterilized and cooled liquid paraffin, grind it into a fine paste, pass through a 200-mesh sieve, and then gradually add sterile and filtered lanolin and yellow petrolatum mixture , mix well, and you get it. All preparation equipment and packaging containers used must be sterilized.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com