A kind of sustained-release sirolimus ophthalmic preparation
A technology of sirolimus and eye drops, which is applied in the direction of aerosol delivery, sensory diseases, and pharmaceutical formulations, etc., can solve problems such as difficulty and difficulty of sirolimus, achieve less toxic and side effects, increase compliance, increase The effect of treatment success
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
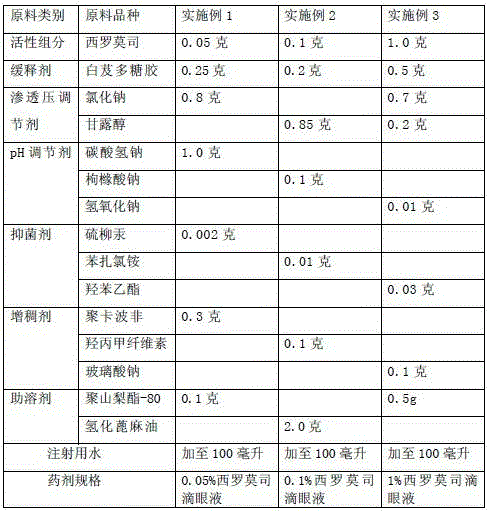
[0020] Example 1-3 Components and dosage of raw materials for preparing sustained-release sirolimus eye drops
[0021]
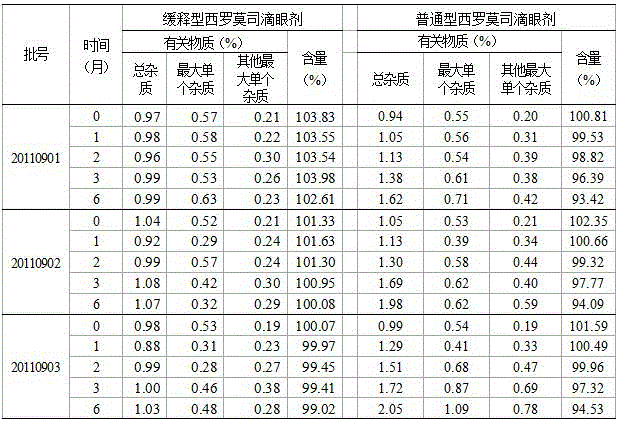
[0022] The bletilla striata polysaccharide gum can be obtained from commercial products or by alcohol-water extraction method: first extract the bletilla striata raw material with 80% ethanol for 5 hours, recover the ethanol from the dregs and extract it with waste water for 3 times, and concentrate the extract under reduced pressure for alcohol extraction. Recover the polysaccharide. The purity of crude bletilla striata polysaccharide gum is generally lower than 80%, the color is slightly yellow, and it has a medicinal smell. It needs to be refined for medicinal purposes. Generally, multiple precipitations with ethanol, washing with ether and acetone organic solvents are used to improve its purity. Ethanol precipitation combined with solvent washing can make the purity of Bletilla striata polysaccharide gum reach 90%-95%. Further improving the p...
Embodiment 4-6
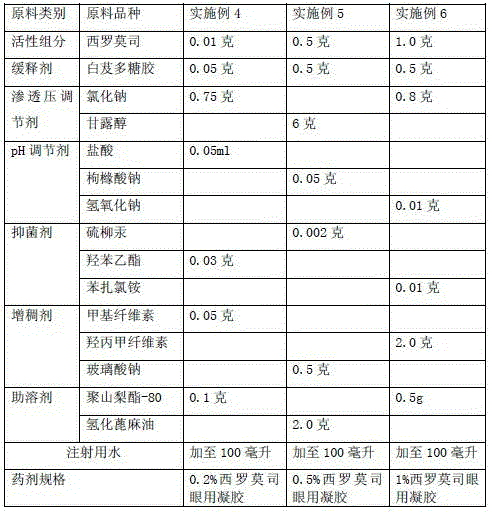
[0025] Example 4-6 Preparation of slow-release sirolimus ophthalmic gel raw material components and dosage
[0026]
[0027] According to the technical scheme of the present invention, the kinds of auxiliary materials that can be selected for preparing sirolimus ophthalmic gel are not limited to the varieties listed in the above table, and the following multiple options can also be selected:
[0028] Wherein, the kind selection and consumption of antibacterial agent are the same as embodiment 1~3.
[0029] The dosage ratio of the thickener to sirolimus is 0.5-5.0:1.0.
[0030] The preparation method is as follows: dissolve the whole amount of sirolimus with bletilla striata polysaccharide glue, dissolve the thickener with water for injection to disperse and let it cool, and then dissolve the pH regulator and bacteriostatic agent with water for injection, and then add the thickener that has been dissolved and sirolimus, supplemented with water for injection to the requi...
Embodiment 7-9
[0031] Example 7-9 Preparation of sustained-release sirolimus ophthalmic ointment raw material components and dosage
[0032] Raw material species Example 7 Example 8 Example 9 Sirolimus 0.2 grams 0.5 grams 1.0g Bletilla striata polysaccharide gum 1 g 2.0 grams 0.5 grams Anhydrous lanolin 10 grams 15 grams 12 grams liquid paraffin 2 grams 8 grams 6 grams Yellow Vaseline 88 grams 76 grams 81 grams Pharmacy specification 0.2% sirolimus eye ointment 0.5% sirolimus eye ointment 1% sirolimus eye ointment
[0033] Preparation method: Dissolve the whole amount of sirolimus with Bletilla striata polysaccharide gum, add appropriate amount of sterilized and cooled liquid paraffin, grind it into a fine paste, pass through a 200-mesh sieve, and then gradually add sterile and filtered lanolin and yellow petrolatum Mixture, mix well, that is. All preparation equipment and packaging containers used must...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com