A kind of sustained release type forsythin eye drops and preparation method thereof
A technology of forsythin eye drops and slow-release type, which is applied in the directions of pharmaceutical formulation, liquid transportation, emulsion transportation, etc., can solve the problems of restricting wide application, difficulty in reaching effective concentration, difficulty in forsythin, etc., and achieve easy raw material The production process is simple and feasible, and the effect of significant economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
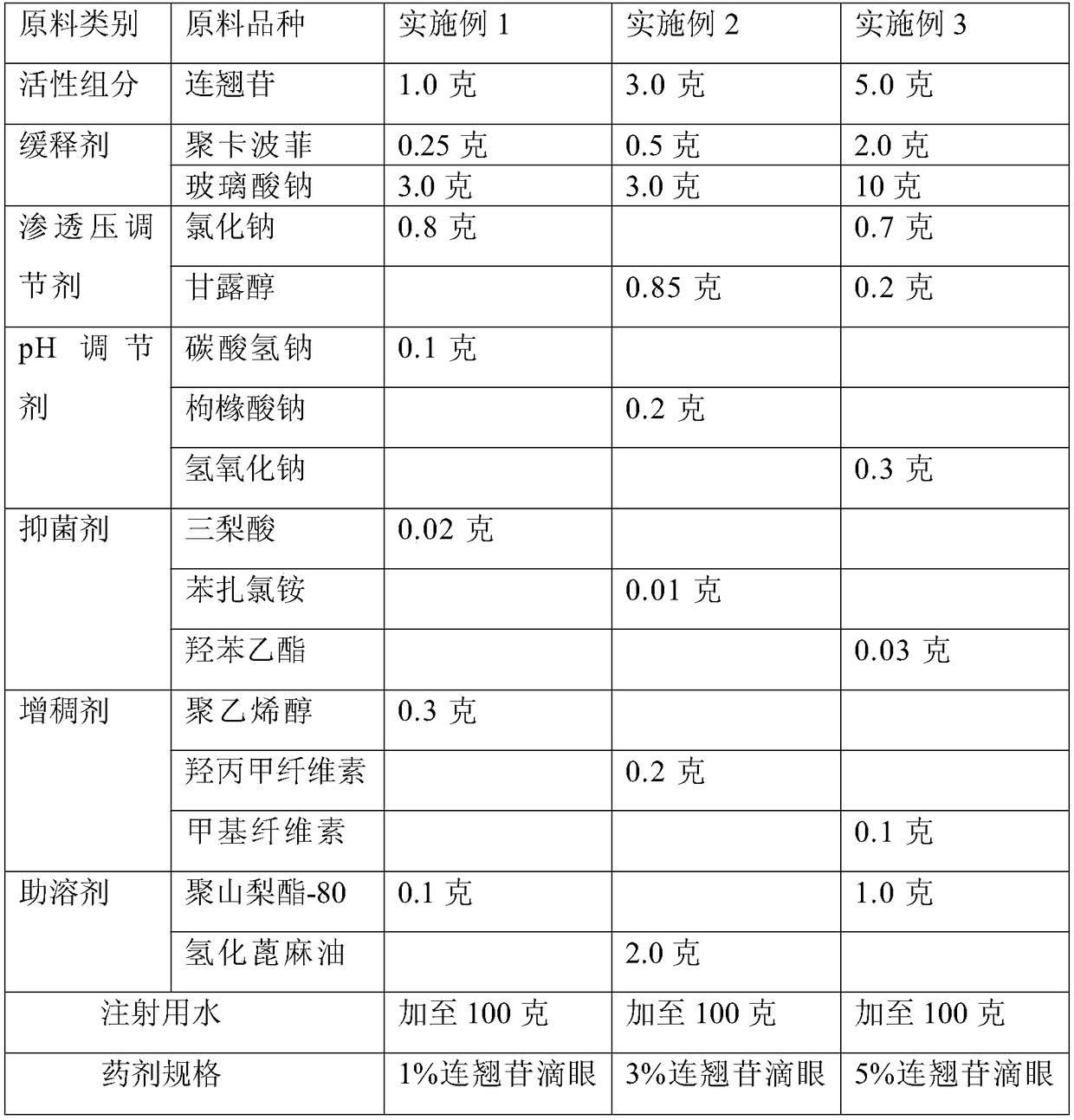
[0035] Prepare raw materials according to the formula shown in table 1,
[0036] Step 1), the full amount of sodium hyaluronate was swelled for 2 hours, polycarbophil was added and stirred to dissolve, and then the full amount of forsythin was dissolved;
[0037] Step 2), disperse the thickener with water for injection and let it cool, and dissolve the pH regulator, osmotic pressure regulator, and bacteriostat with water for injection, stir well and filter, and combine the above two parts of the mixed solution;
[0038] Step 3), add dissolved forsythin to the mixed solution obtained in step 2), add water for injection to the full amount, filter, sub-package, and obtain, the pH value of the obtained eye drops is 5.5-7.5, drop The osmolality of the eye solution is 250-350 mOsmol / kg.
[0039] Table 1 Preparation of sustained release type forsythin eye drops raw material components and dosage
[0040]
[0041]
experiment example 2
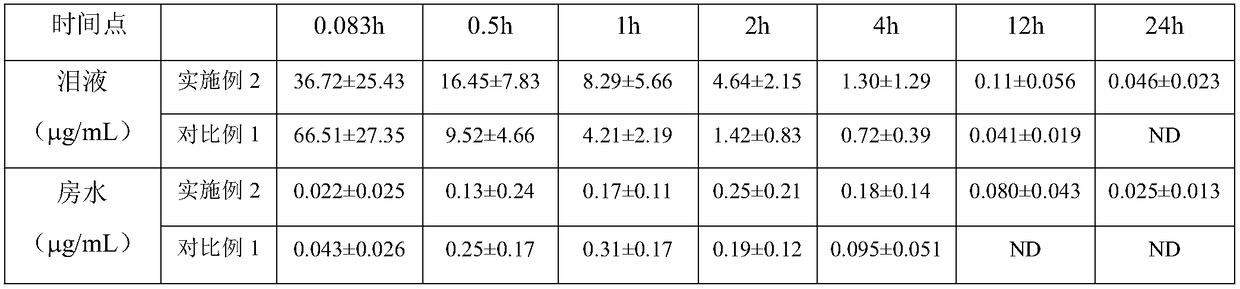
[0042] Pharmacokinetic test of experimental example 2 forsythin sustained-release eye drops
experiment example 3
[0067] Experimental Example 3 Eye Irritation Test of Sustained-release Forsythin Eye Drops with Different Concentrations
[0068] The eye drops prepared by the method of Example 1-3 of the present invention were used for eye irritation test.
[0069] Comparative Example 2: Dexamethasone Eye Drops (0.1%)
[0070] 24 healthy New Zealand rabbits (half male and half female) (provided by Qingdao Kangda Biotechnology Co., Ltd.). The eyes of the animals were checked 24 hours before the experiment, and animals with normal eyes, no inflammation, no defects, and no old corneal damage were selected for the experiment. The self-comparison method was used to measure the left and right sides of the same body. Drugs were given to the left eye, 0.9% sodium chloride injection was given to the left eye of the negative group, and the right eye was used as a negative control. 100 μL per eye, once a day, for 7 consecutive days. Before administration every day and 1, 2, 4, 24, 48 and 72 hours aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com