Slow release type linezolid medicament for eyes and preparation method thereof
A technology of linezolid ophthalmic and linezolid, which is applied in the field of slow-release linezolid ophthalmic medicine and its preparation, can solve the problems of poor solubility and stability of linezolid, achieve simple and feasible preparation process, increase treatment The effect of high success rate and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
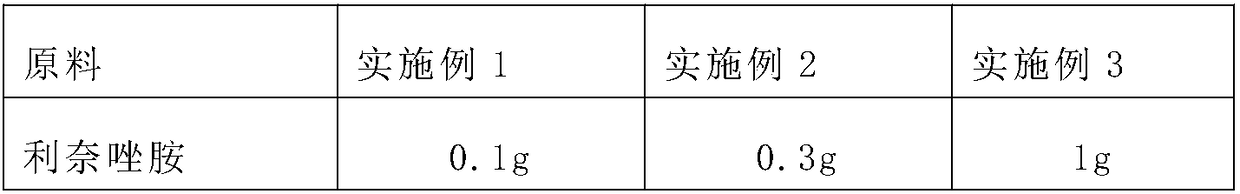
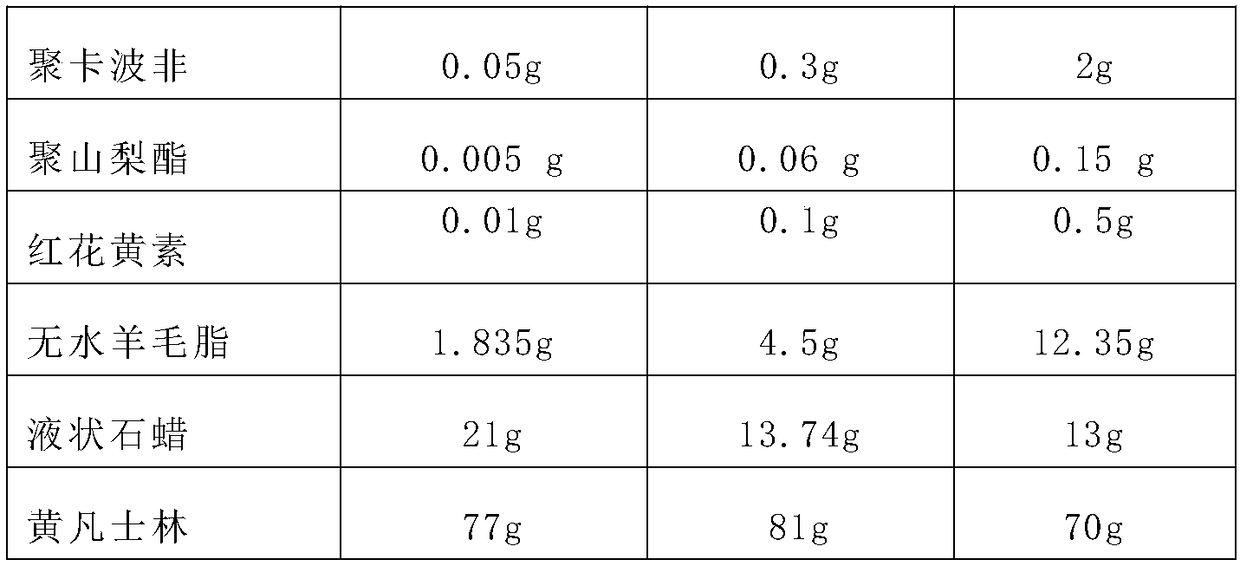
[0019] A sustained-release linezolid eye ointment, comprising components: linezolid, polycarbophil, polysorbate, safflower flavin, anhydrous lanolin, liquid paraffin and yellow petrolatum, and the mass fraction ratio of each component is Linezolid: polycarbophil: polysorbate: safflower flavin: anhydrous lanolin: liquid paraffin: yellow petrolatum = 1:0.5~2:0.05~0.2:0.01~0.5:8~15:2~ 10:75-95.
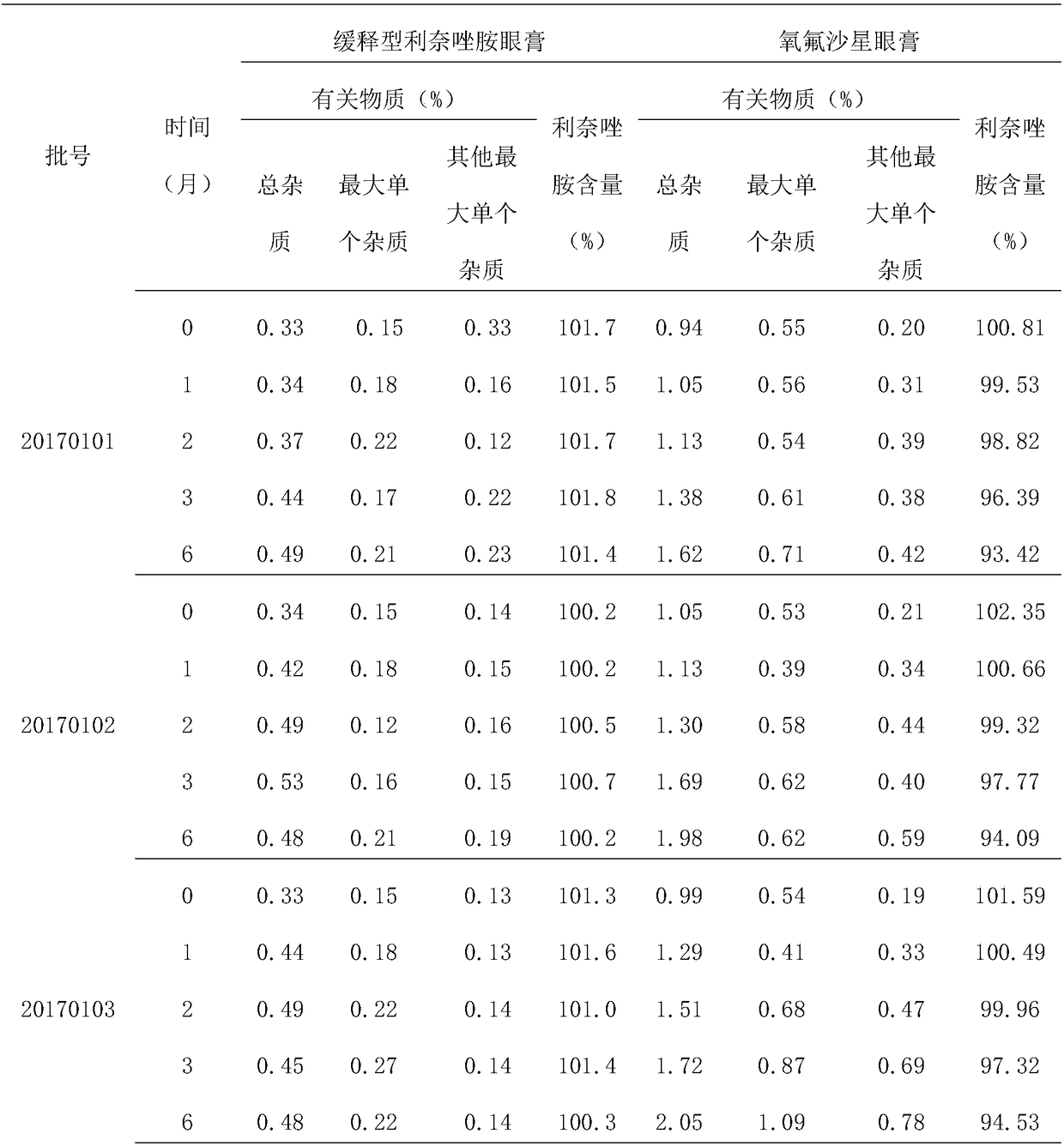
[0020] The above-mentioned ophthalmic drugs are prepared with linezolid as the main active ingredient, safflower flavin, polycarbophil and polysorbate as the mucoadhesive polymer drug delivery system, and prepared with excipients. release formulations.
[0021] Among them, the raw materials and proportions of the sustained-release linezolid ophthalmic drugs in each of Examples 1 to 3 are shown in Table 1:
[0022] Table 1 The raw materials and proportioning situation of the sustained-release linezolid eye ointment of Examples 1-3
[0023]
[0024]
[0025] The preparation method...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com