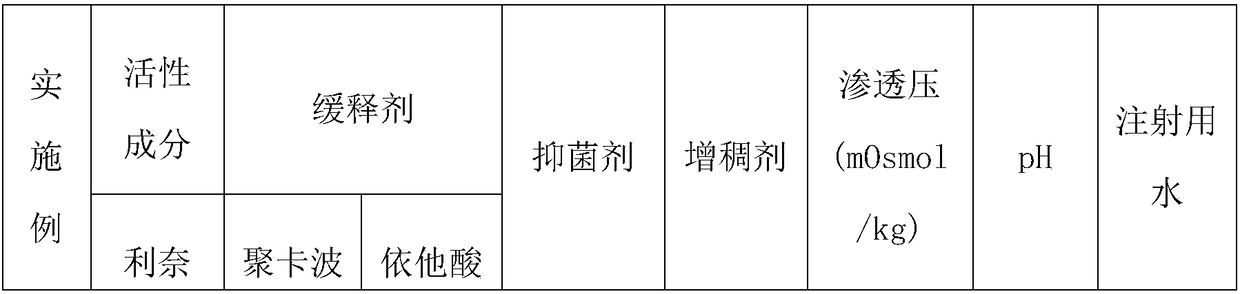
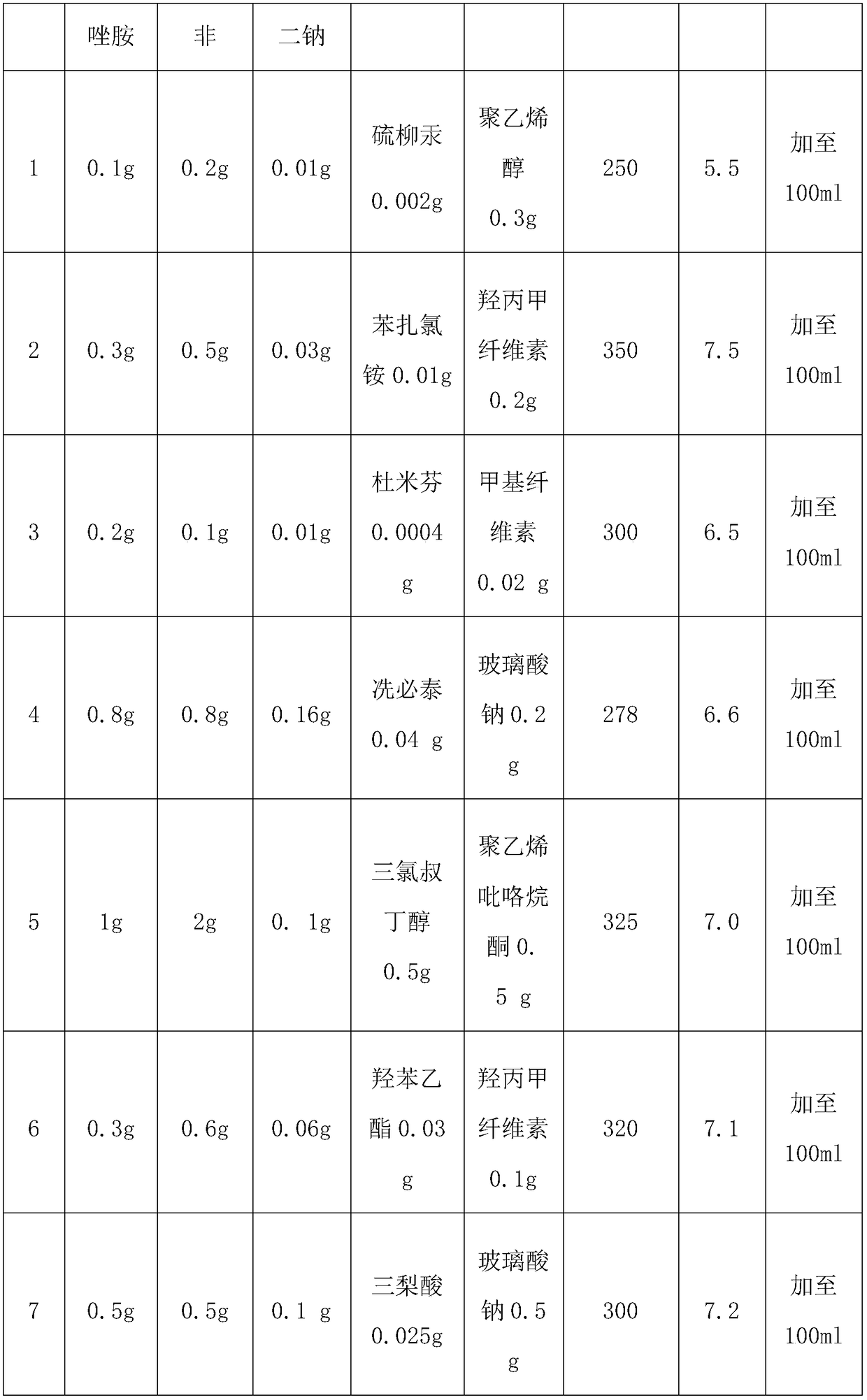
Slow release type linezolid preparation for eyes as well as preparation method and application thereof
A linezolid eye and linezolid technology are applied in the field of sustained-release linezolid ophthalmic preparations and their preparation, which can solve the problems of increasing the incidence of infectious endophthalmitis and the like, and achieve high intraocular bioavailability, The effect of strong targeting effect and good intraocular penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 8
[0032] (1) Embodiment 8 pharmacokinetic test
[0033] Healthy New Zealand rabbits (half male and half female) were used as experimental animals. A single dose of linezolid eye drops (at 20, 40, 60, 80, 100, 120, 150, 210, 270, 360, 480 minutes after administration, the aqueous humor 30 μl) and multiple times (7 days of continuous administration, 4 times a day, once every 6 hours. Take 30 μl of aqueous humor before the last administration on the 6th day, before the 1st and 2nd administration on the 7th day, At 20, 40, 60, 80, 100, 120, 150, 210, 270, 360, 480 min after the third administration, 30 μl of aqueous humor was extracted) After administration, the aqueous humor samples were determined by HPLC-MS.
[0034]After a single administration, the Cmax of linezolid in aqueous humor was 38.754±9.426g / ml; Tmax was 102.00±12.55min; T1 / 2 was 81.52±13.64min; AUC0-t was 5179.60±1881.16gminml-1, AUC0- was 5374.01±2056.85gminml-1; Ke is 0.01±0.001min-1. After repeated eye drops, the...
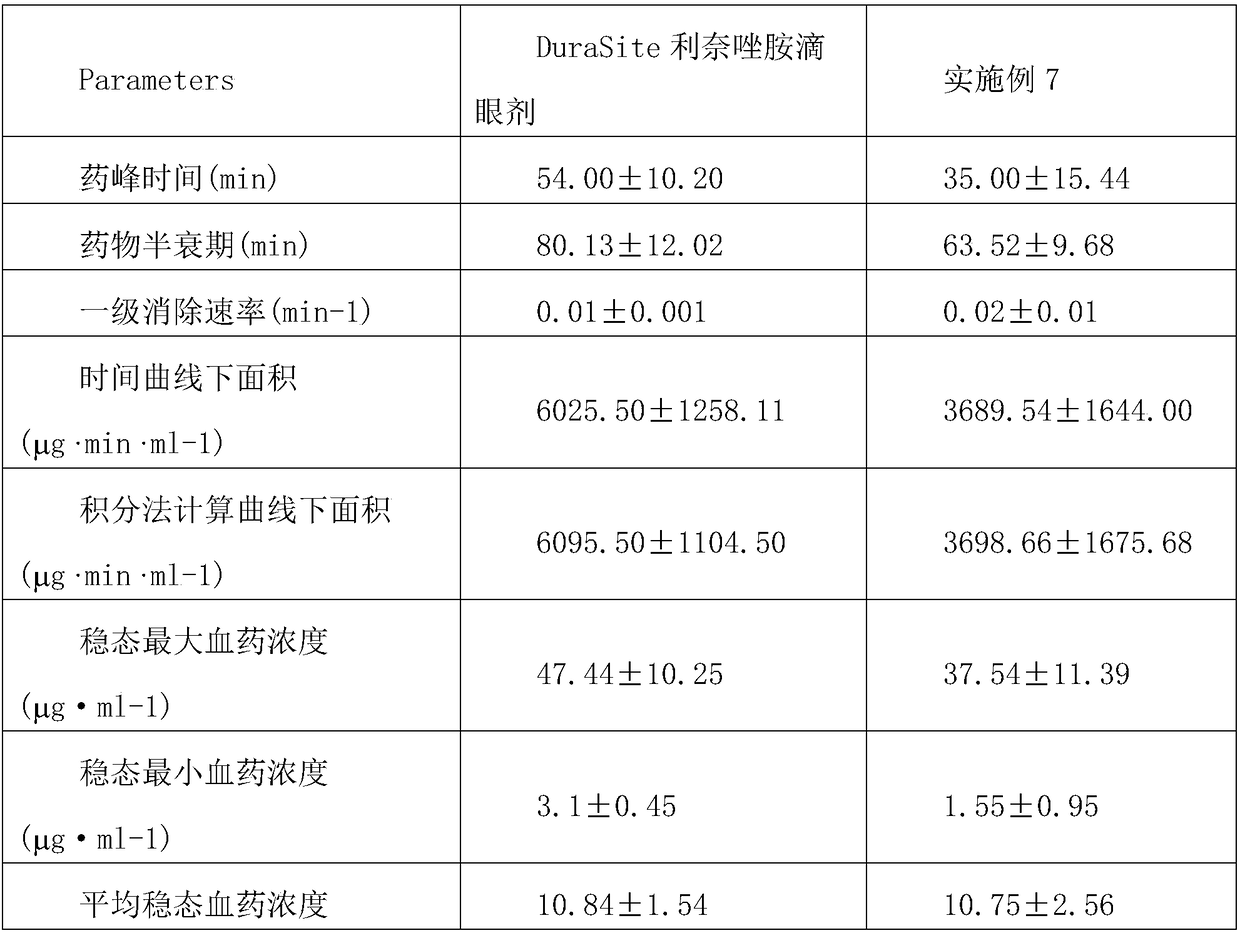
Embodiment 7
[0053] Pharmacokinetic test of linezolid eye drops prepared from sustained-release linezolid eye drops and DuraSite ophthalmic delivery system in Example 7
[0054] The left and right eyes of New Zealand rabbits were administered at the same time, and the dosage was 50 μL. At 20, 40, 60, 80, 100, 120, 150, 210, 270, 360, and 480 minutes after administration, 30 μl of aqueous humor was extracted for testing; aqueous humor samples were determined by LC-MS, and the results are shown in Table 4 below:
[0055] Table 4: The main pharmacokinetic parameters of the sustained-release linezolid eye drops and DuraSite linezolid eye drops in Example 7 administered to the eyes of New Zealand rabbits in a single dose
[0056]
[0057]
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com