Patents
Literature
46 results about "Intraocular Injections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
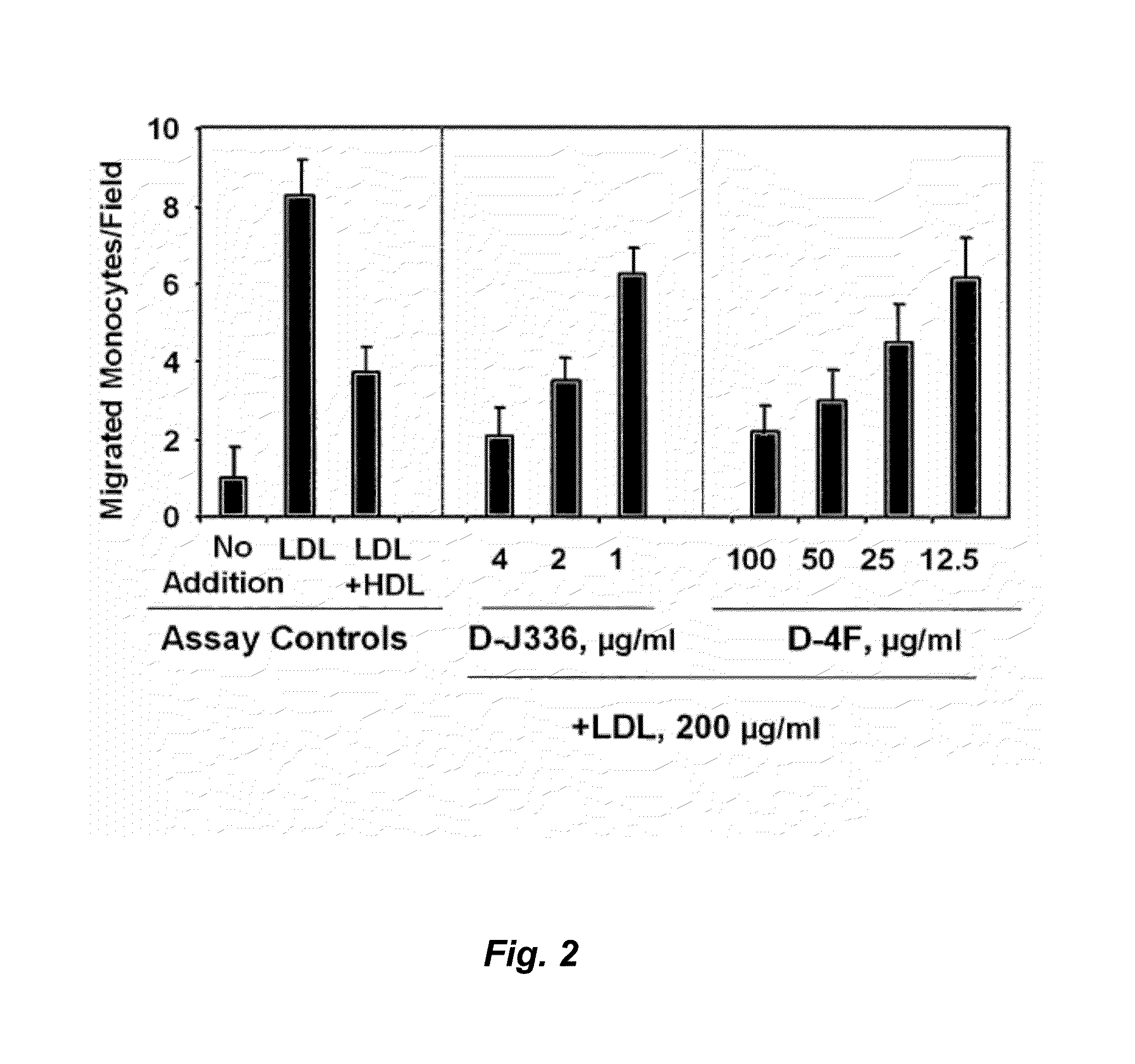
Intraocular injection. The injection of medications such as steroids, Lucentis®, or Avastin® into the eye has become common in the treatment of several retinal diseases, particularly diabetic retinopathy, macular degeneration, and retinal vein occlusion.
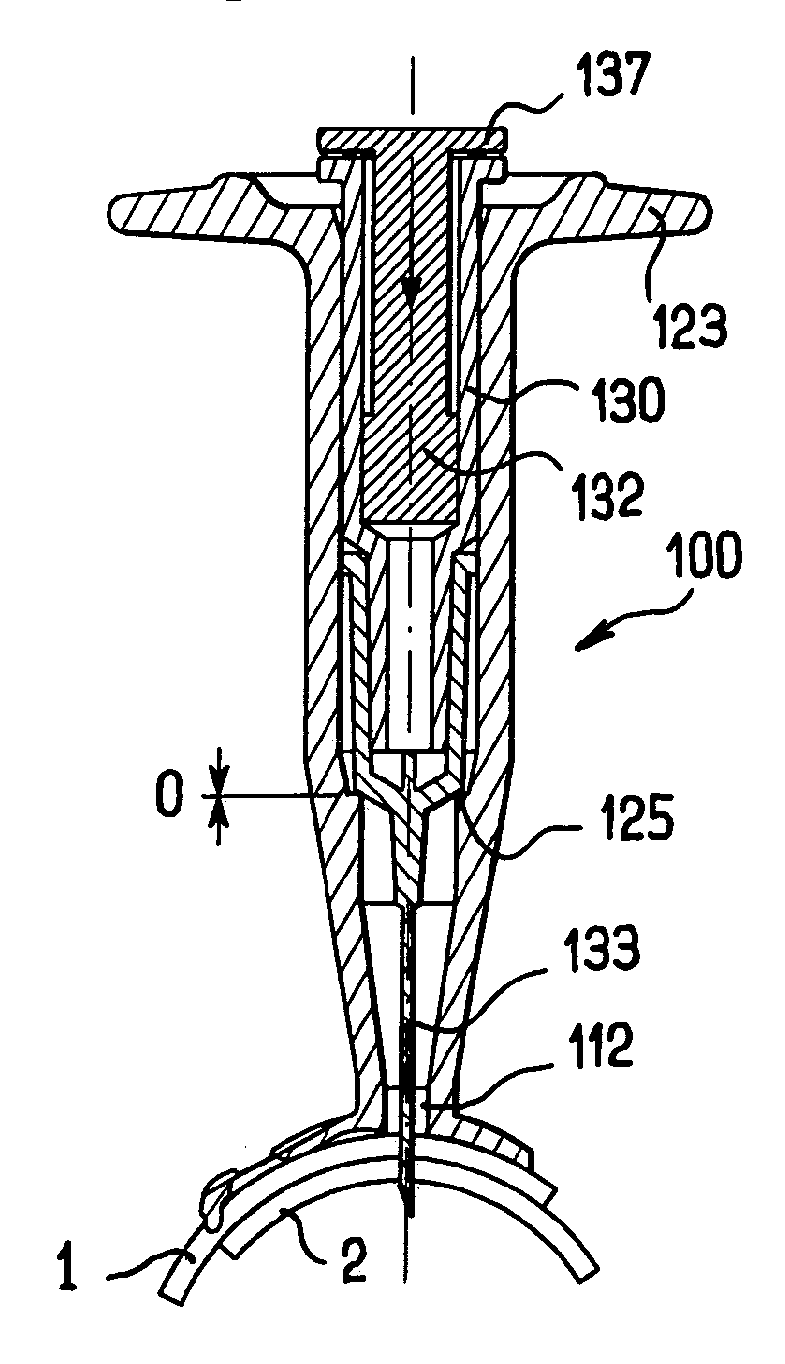
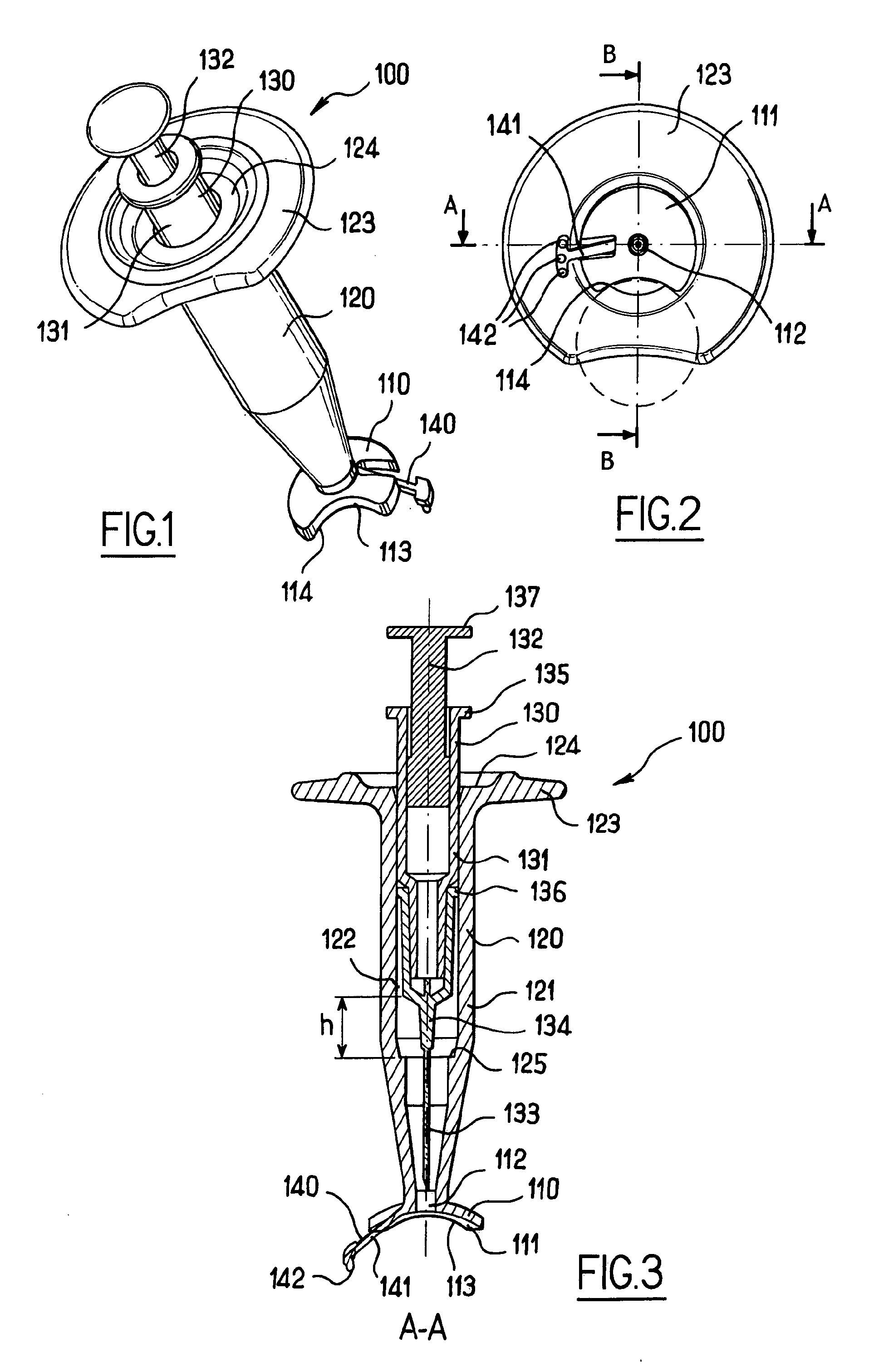
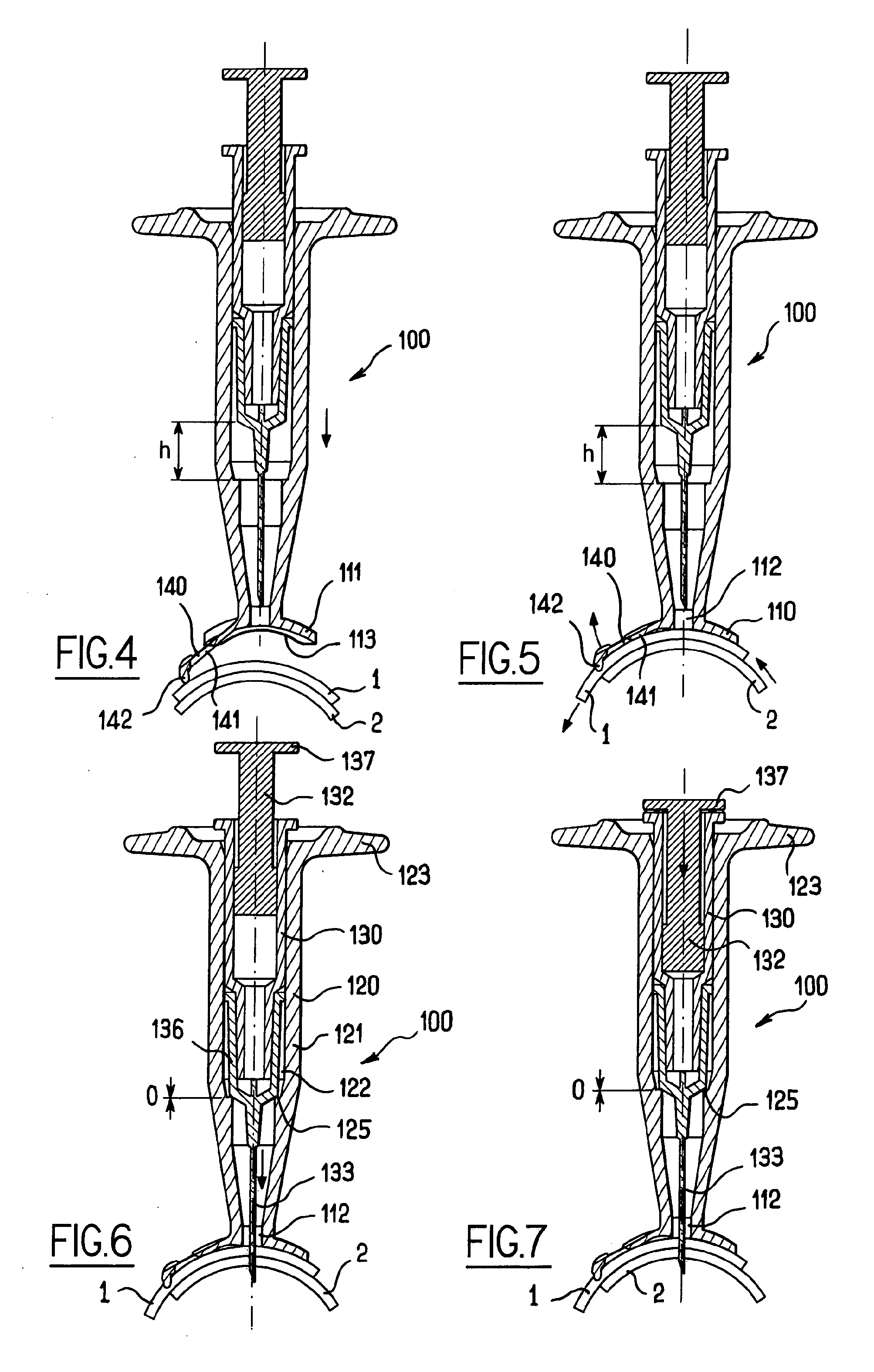
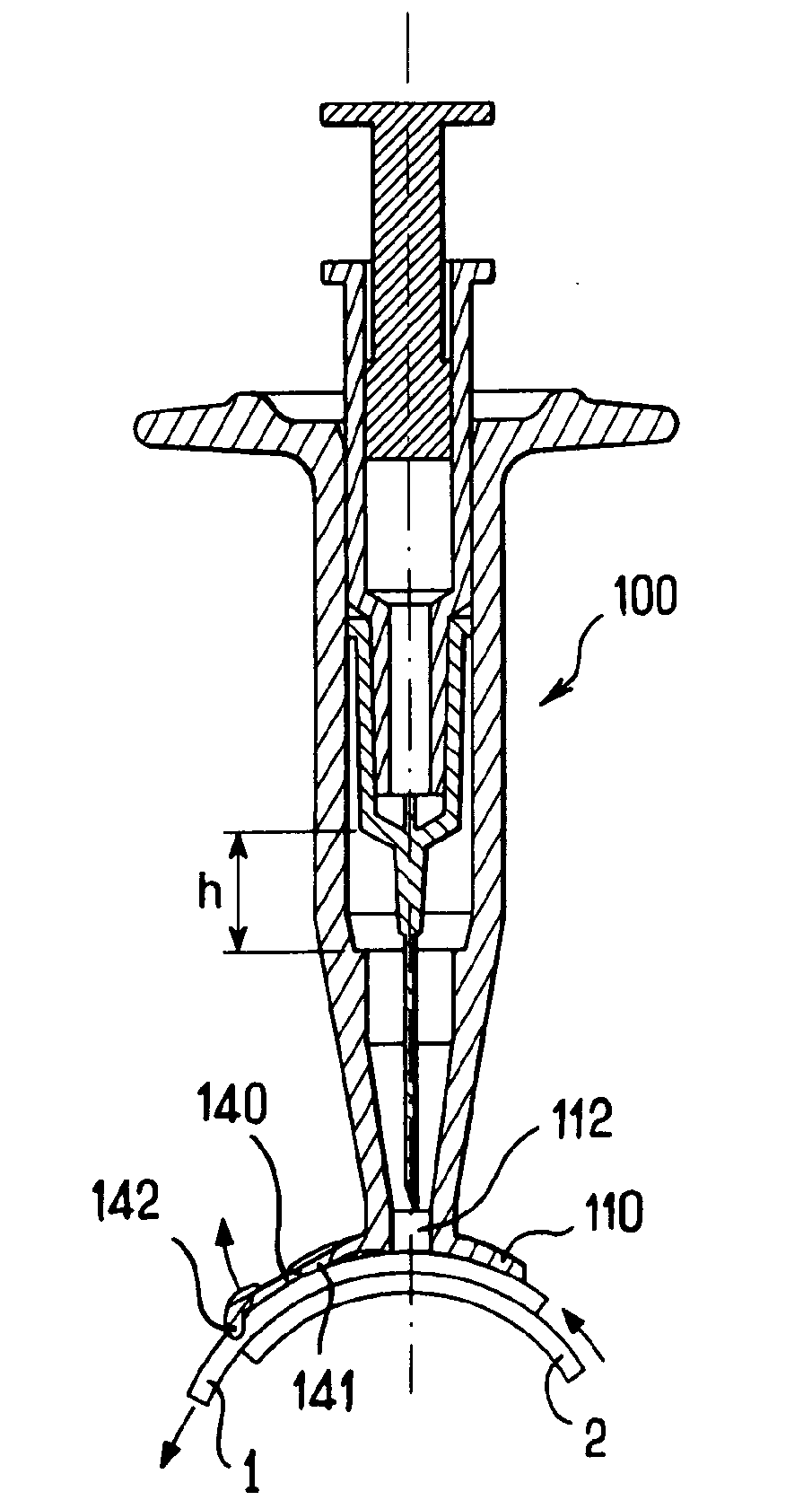
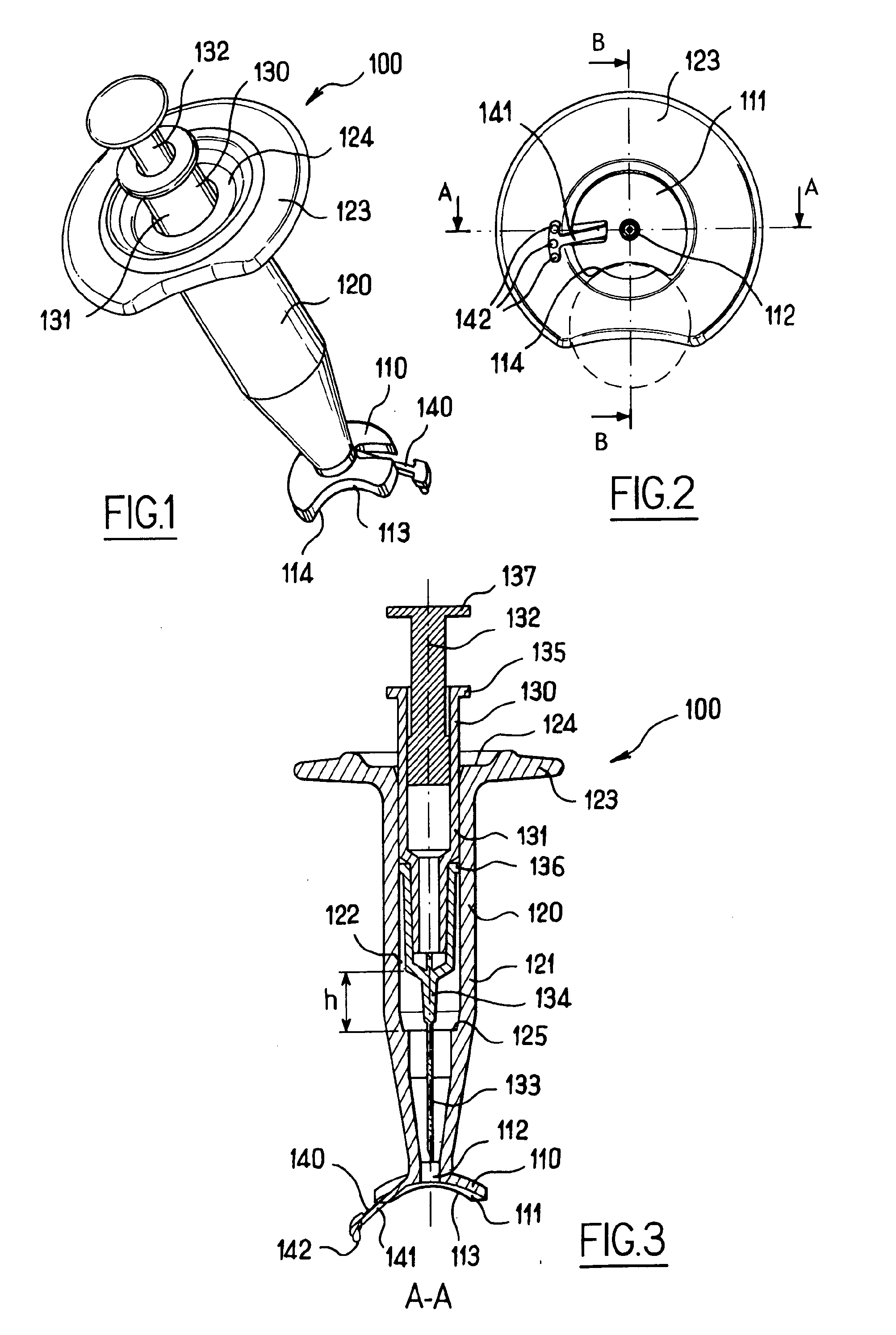
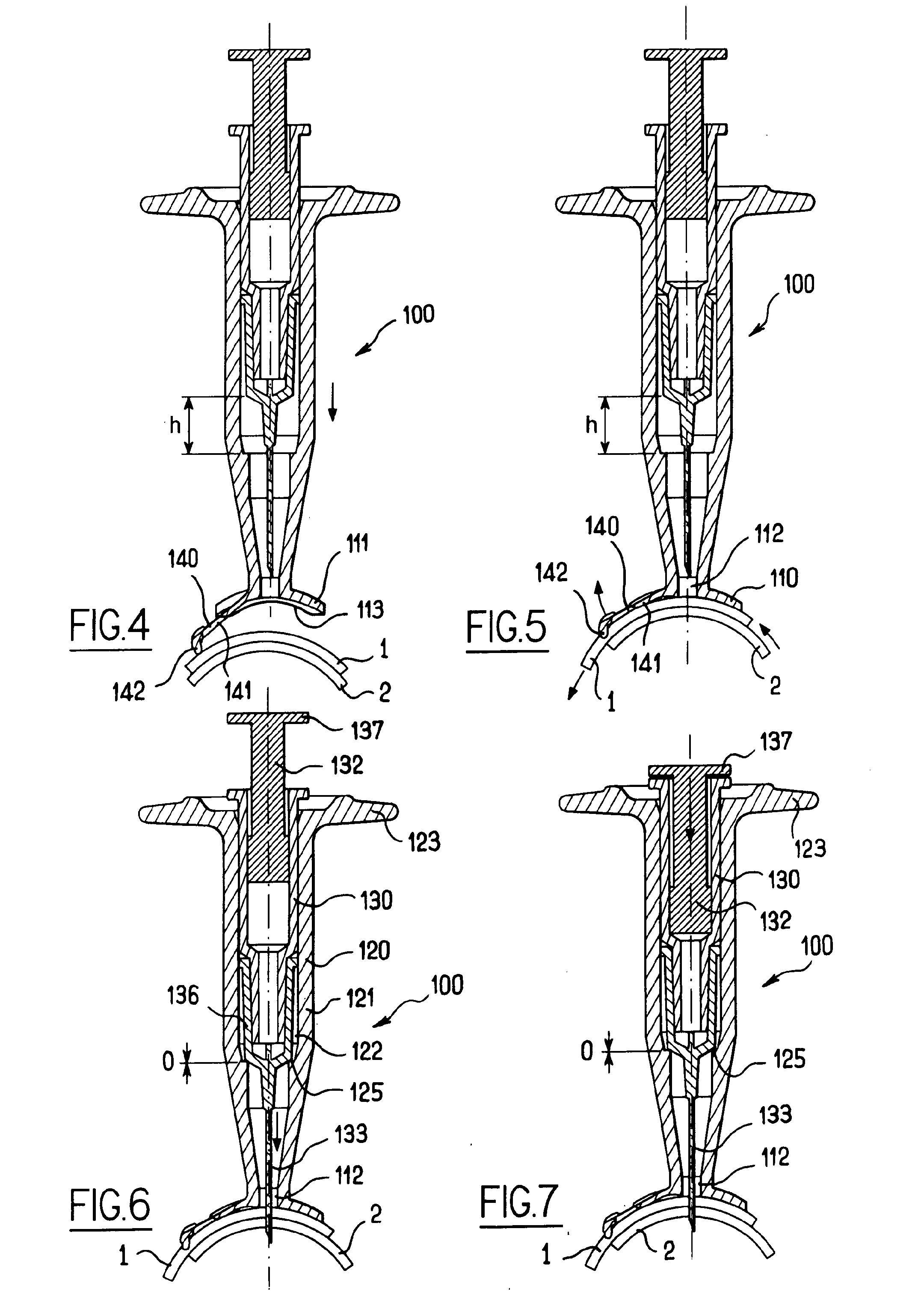
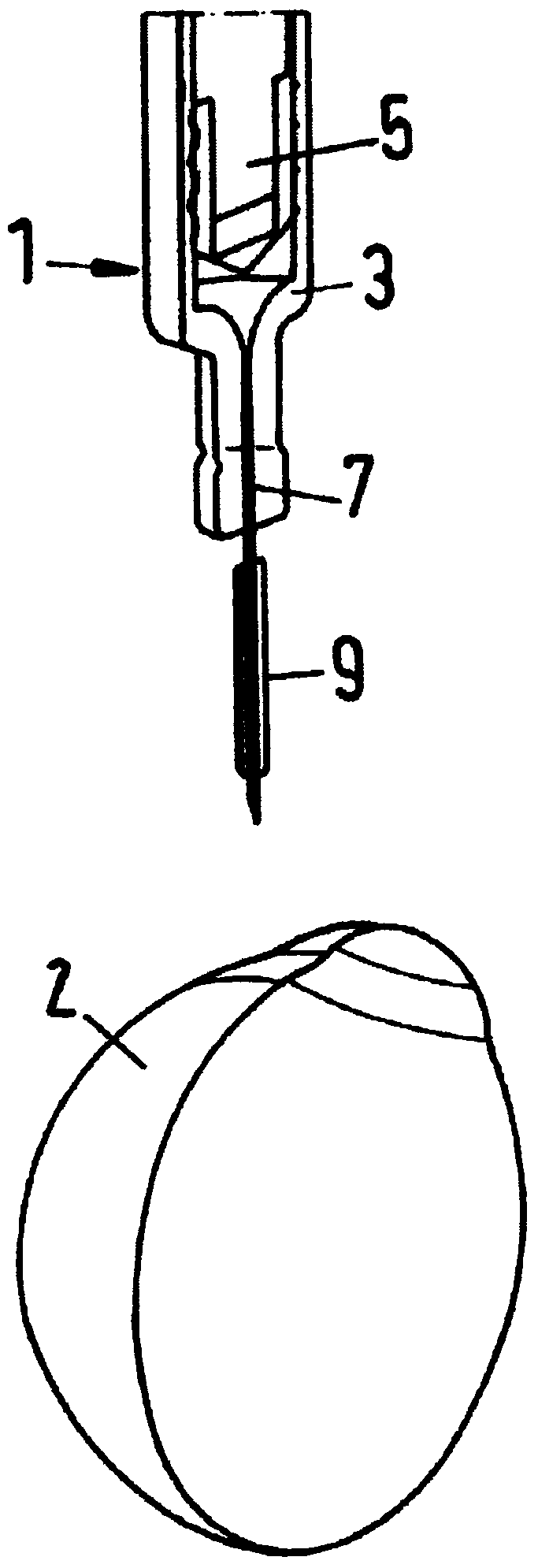
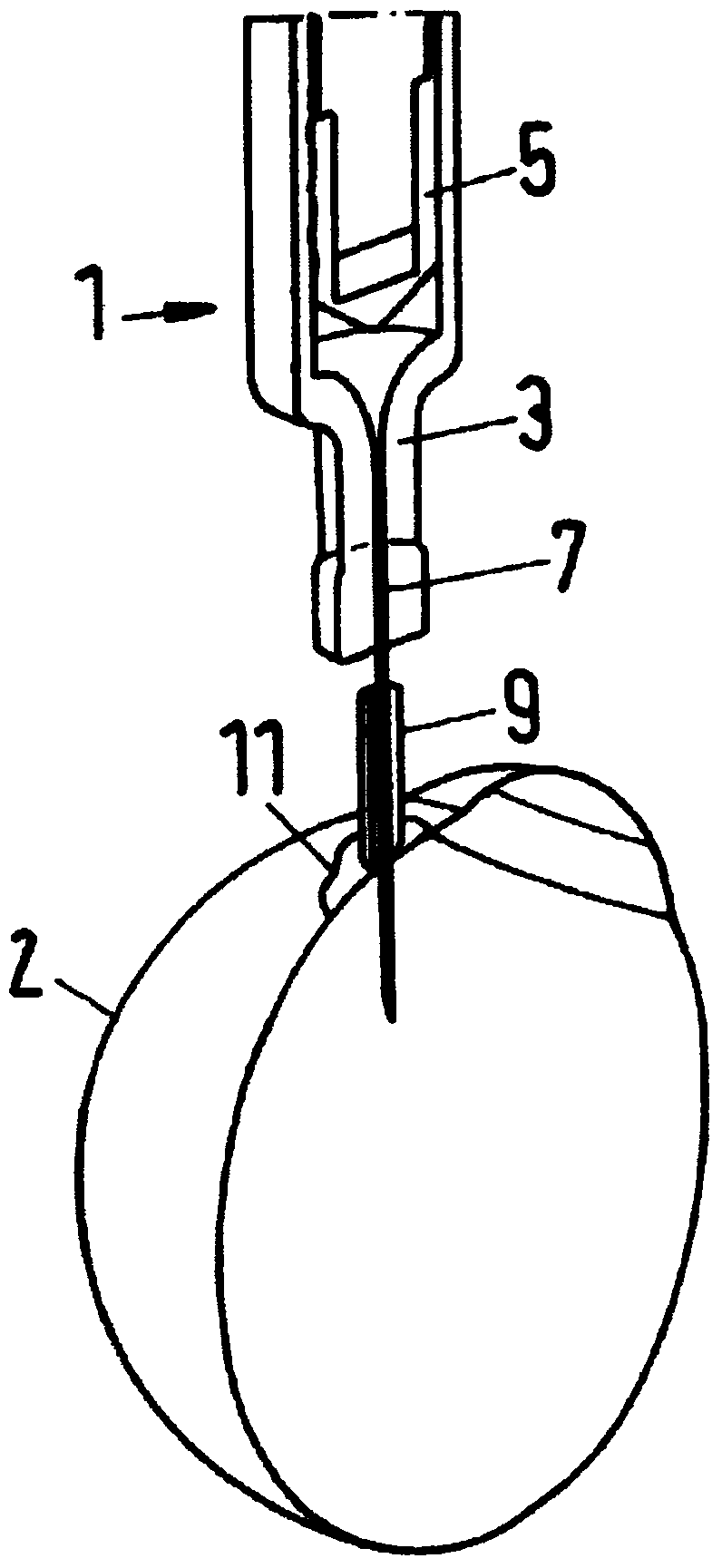
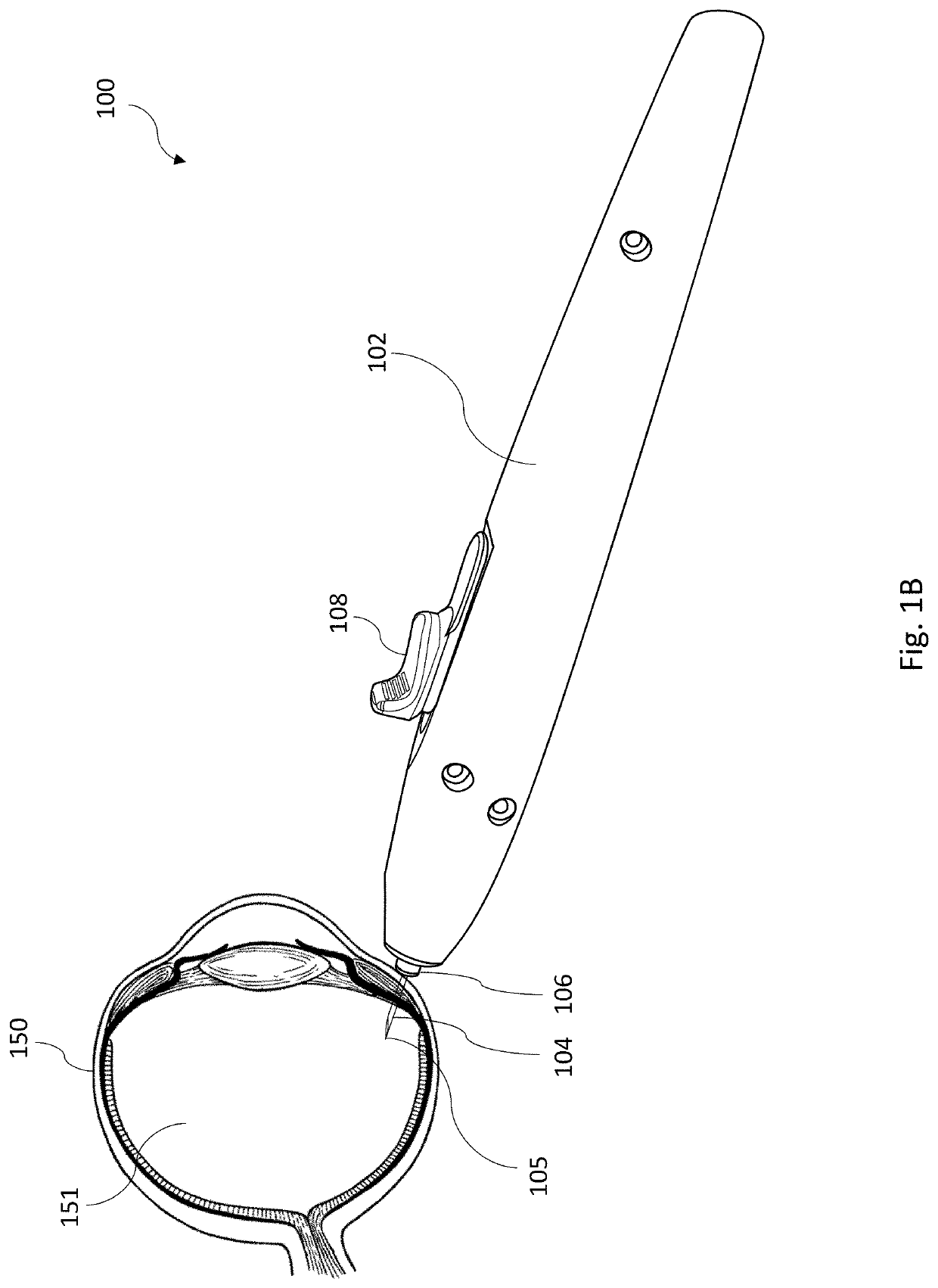
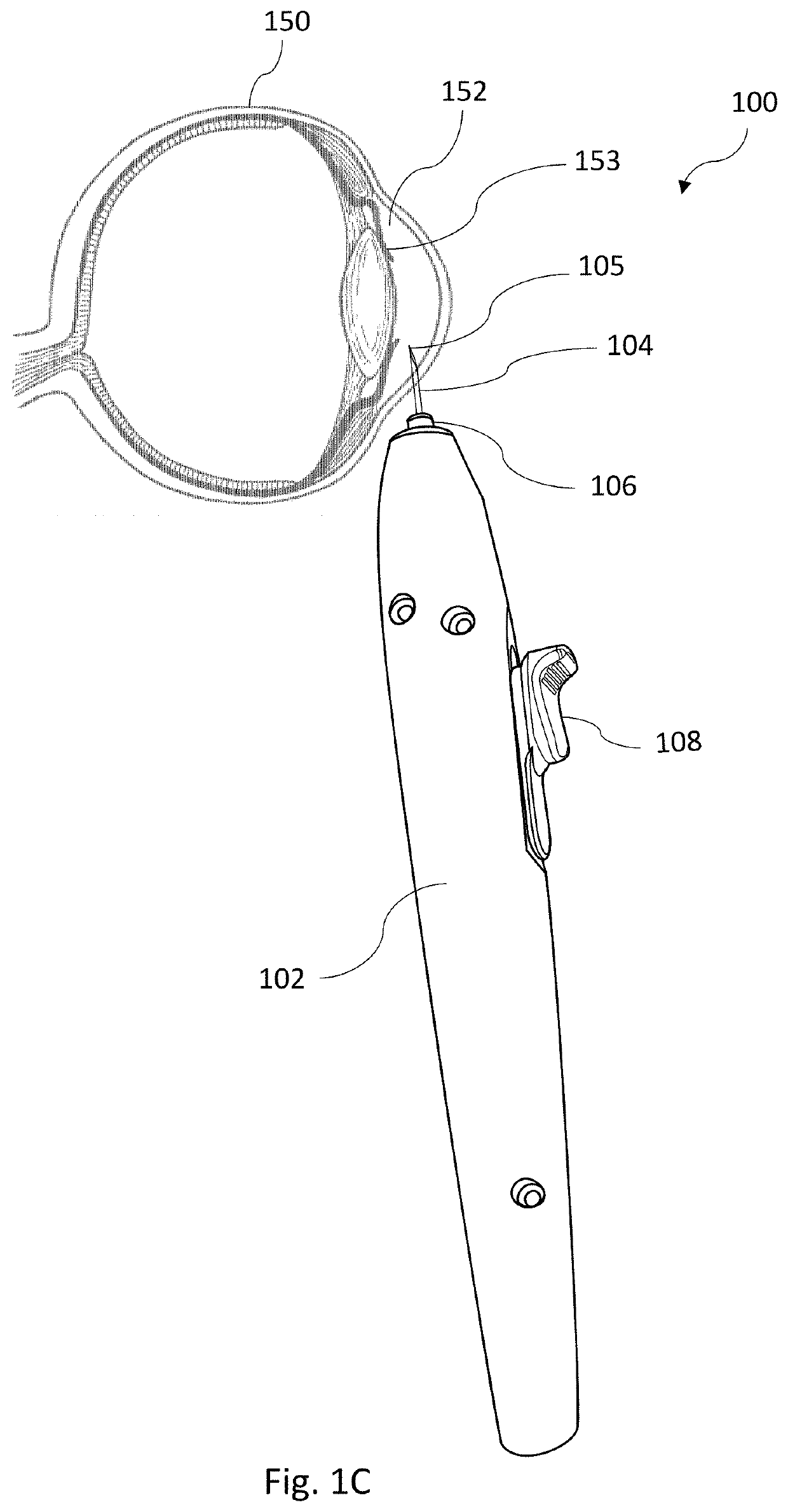
Apparatus for intra-ocular injection
ActiveUS20100010452A1Easy to handleLimit risk of leakageInfusion syringesEye treatmentOphthalmologyIntraocular Injections
Owner:FOVEA PHARMA +1
Apparatus for intra-ocular injection
The application relates to an apparatus for intraocular injection comprising a plate adapted for being brought into contact with an eye and guiding means for guiding a needle into the interior of an eye, characterized in that the plate comprises a cut-out having an edge adapted to be positioned along the limbus delimiting the cornea and the sclera of the eye, so as to adjust the position of the guiding means with respect to the limbus.
Owner:FOVEA PHARMA +1
Treatment of ocular disease
InactiveUS7354574B2Without substantial ocular toxicityHindering progressSenses disorderMetabolism disorderDiabetic retinopathyDisease
A method and article to treat ocular disease with Cyclosporin A alone or with compounds related to Cyclosporin A for intraocular injection or implantation. Treatment does not result in ocular toxicity and encompasses age related macular degeneration, retinitis pigmentosa, and retinopathy such as diabetic retinopathy.
Owner:PEYMAN GHOLAM A DR
Long-acting sustained release preparation for preventing or treating retinal damage, and preparation method thereof
ActiveCN102233129ARelieve painSolve the problem of long-term frequent injectionsSenses disorderPeptide/protein ingredientsMicrosphereRetinal ganglion
The invention belongs to the field of pharmaceutical preparations, and relates to a long-acting sustained release preparation for preventing or treating retinal damage, and a preparation method thereof. The long-acting sustained release preparation takes erythropoietin (EPO) as an active component, takes dextran as a protective agent for the active component, and takes poly(lactic-co-glycolic acid), polylactic acid or polycaprolactone as a coating component to prepare sustained release microspheres, wherein the erythropoietin is coated by the poly(lactic-co-glycolic acid), polylactic acid or polycaprolactone. Proven by animal experiments, the sustained release microspheres of the long-acting sustained release preparation provided by the invention have the same protective actions on the ganglionic cells of damaged retinas by single vitreous chamber injection and repeated EPO protein vitreous chamber injection, and the sustained release microspheres are capable of avoiding a series of complications caused by many times of injection and overcoming the defects of repeated intraocular injection administration and gene therapy. The long-acting sustained release preparation provided by the invention adopts intraocular local administration, can reduce the dosage and treatment cost, and can not generate adverse effects on other organs or tissues in vivo.
Owner:SHANGHAI JIAO TONG UNIV +1
Application of in-situ crosslinking hydrogel capable of intraocular injection in preparing artificial vitreous bodies
The invention discloses an application of in-situ crosslinking hydrogel in preparing artificial vitreous bodies, which is characterized in that the in-situ crosslinking hydrogel is prepared by in-situ crosslinking after mixing a four-arm-end mercapto polyethylene glycol solution shown in the formula (I) and a polymer solution shown in the formula (II); wherein m in the formula (I) is an integer larger than 1, and n in the formula (II) is an integer larger than 1; and the two solutions are filled in eyes without vitreous bodies after mixing and can effectively form gel. A cell experiment and an animal in vivo experiment prove that the in-situ crosslinking hydrogel does not cause obvious inflammatory reaction and intraocular organ toxic reaction and can be used as the artificial vitreous bodies.
Owner:PEOPLES HOSPITAL PEKING UNIV
Long-acting sustained release preparation for preventing or treating retinal damage, and preparation method thereof
ActiveCN102233129BImprove survival rateLong-term protectionSenses disorderPeptide/protein ingredientsMicrosphereRetinal ganglion
Owner:SHANGHAI JIAOTONG UNIV +1
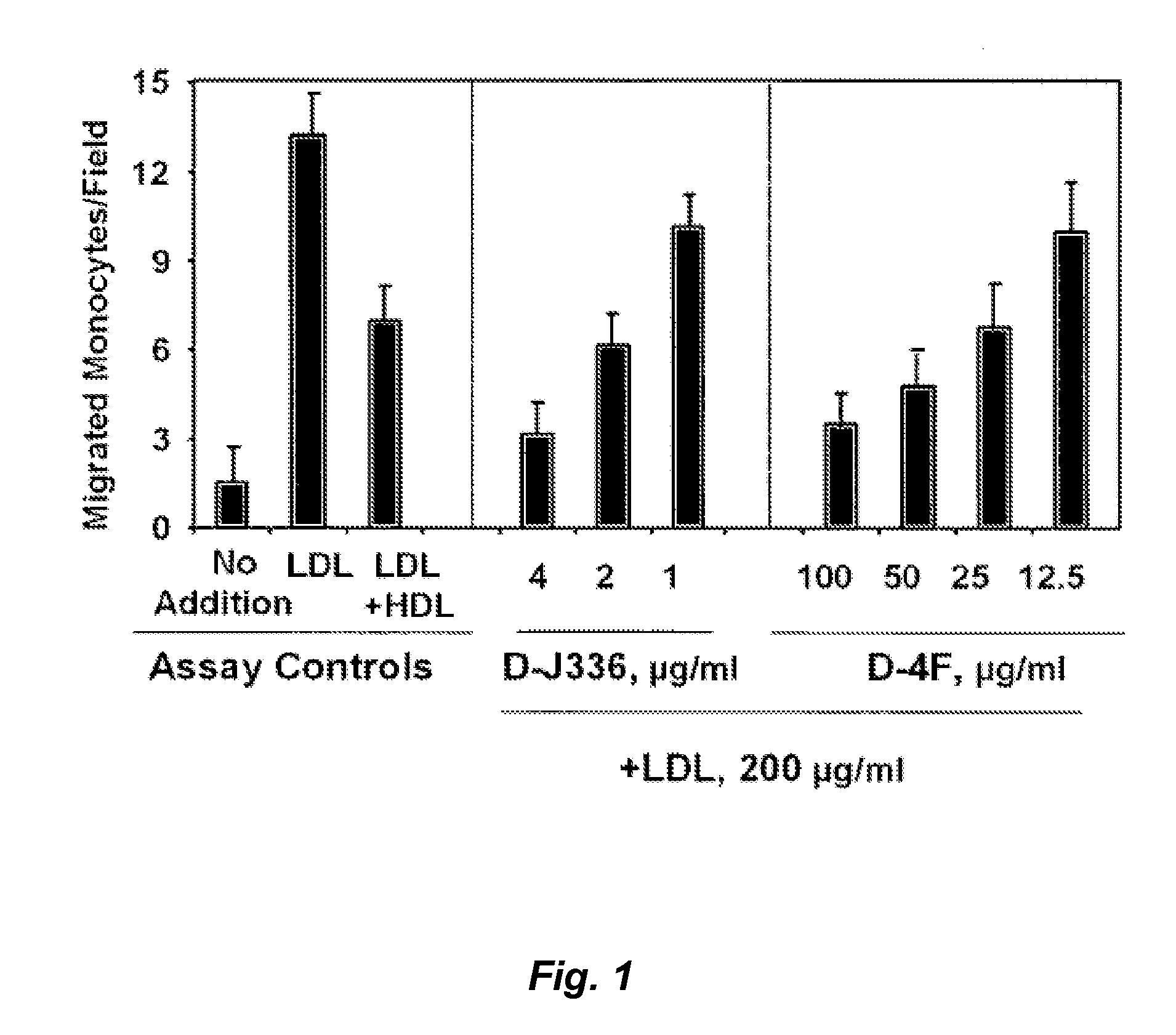
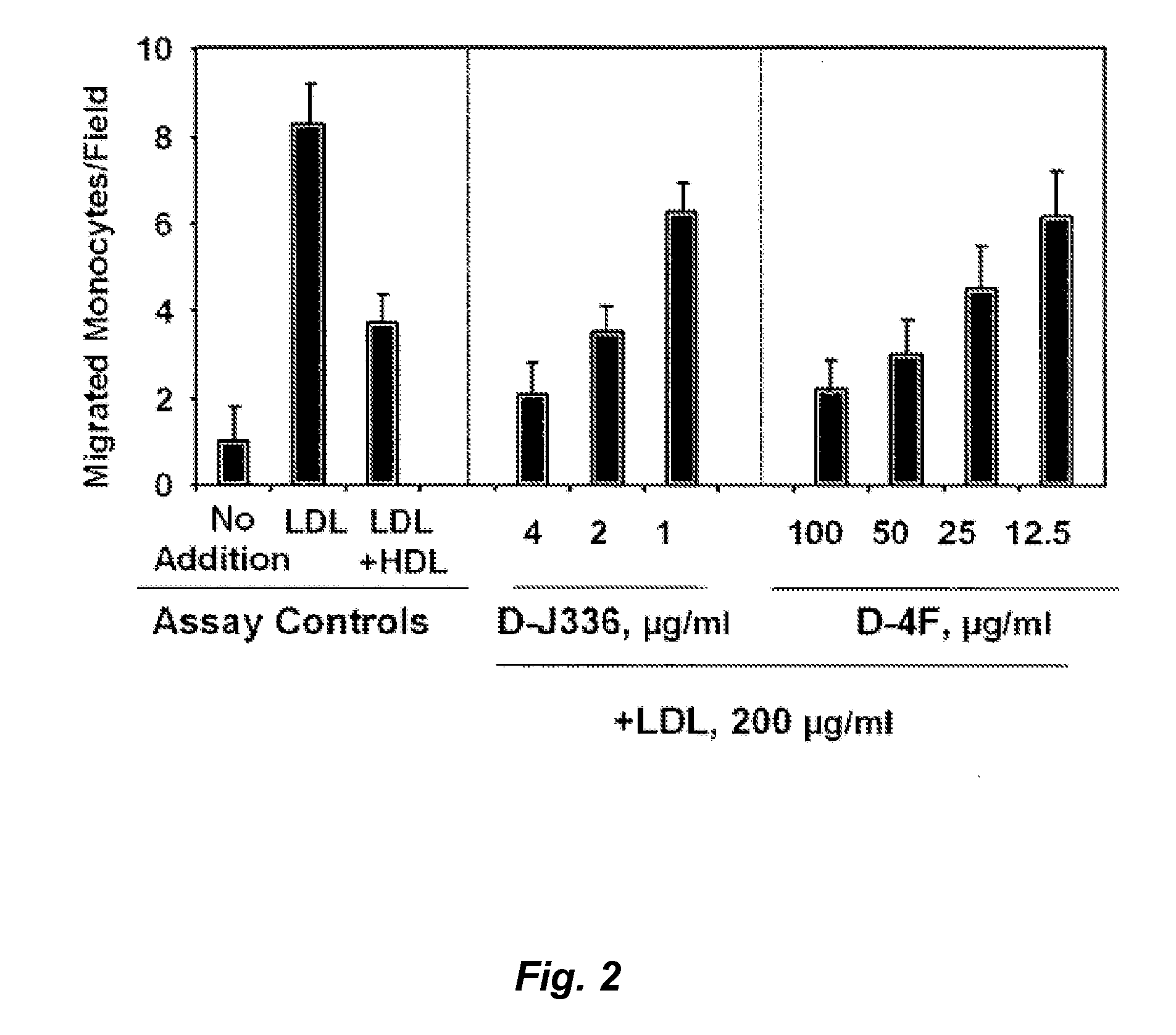
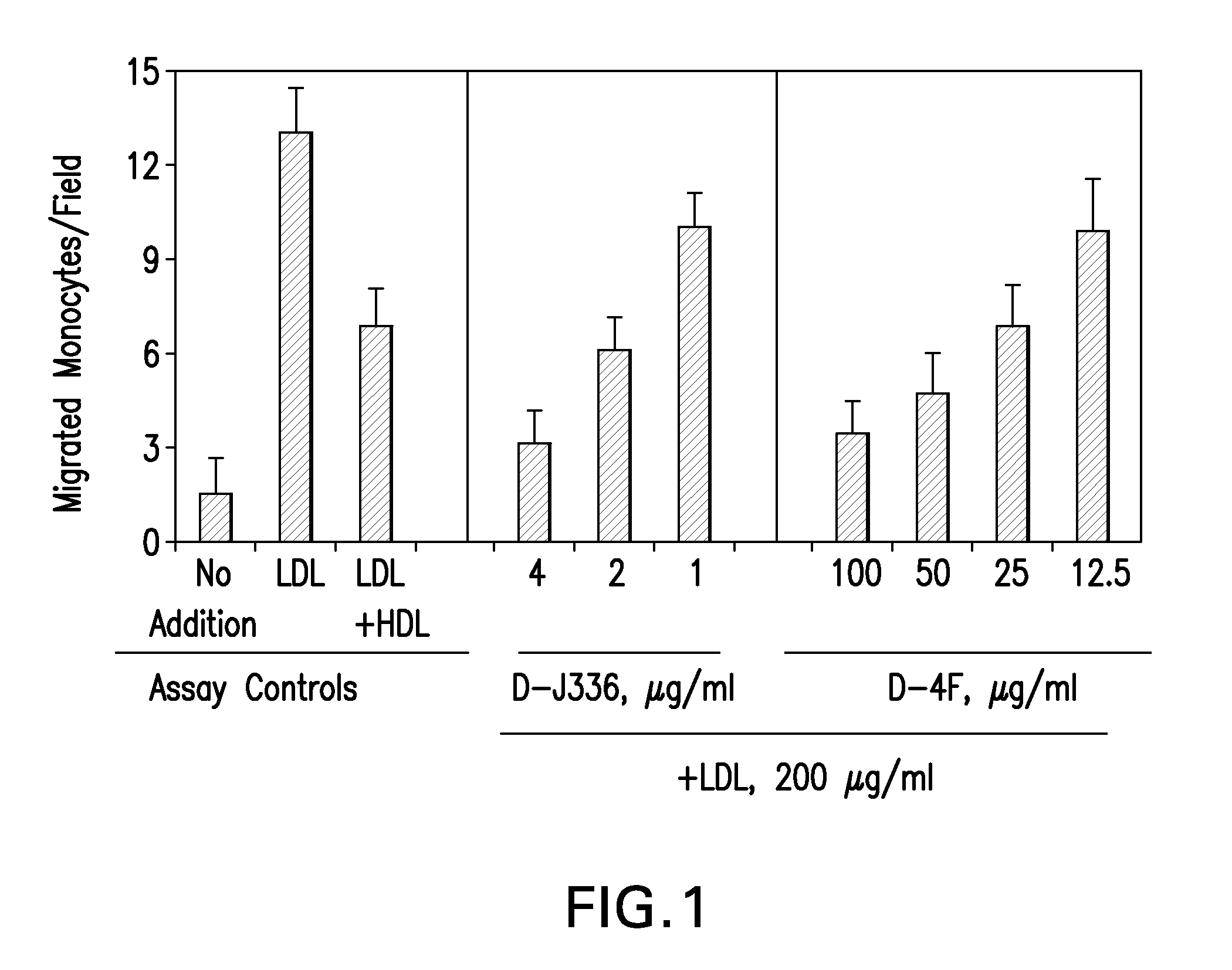
Peptides and peptide mimetics to treat pathologies associated with eye disease
Owner:RGT UNIV OF CALIFORNIA
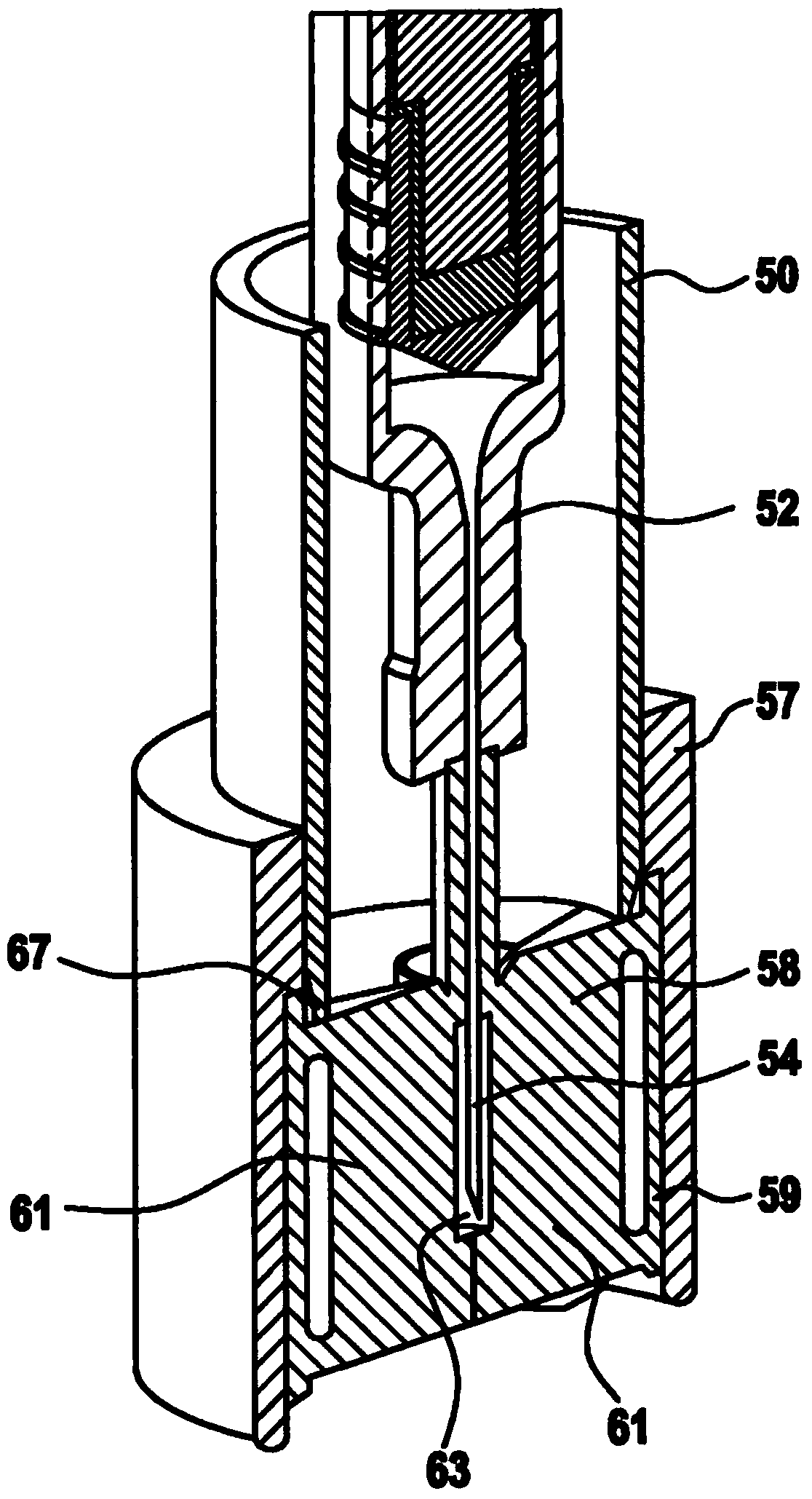
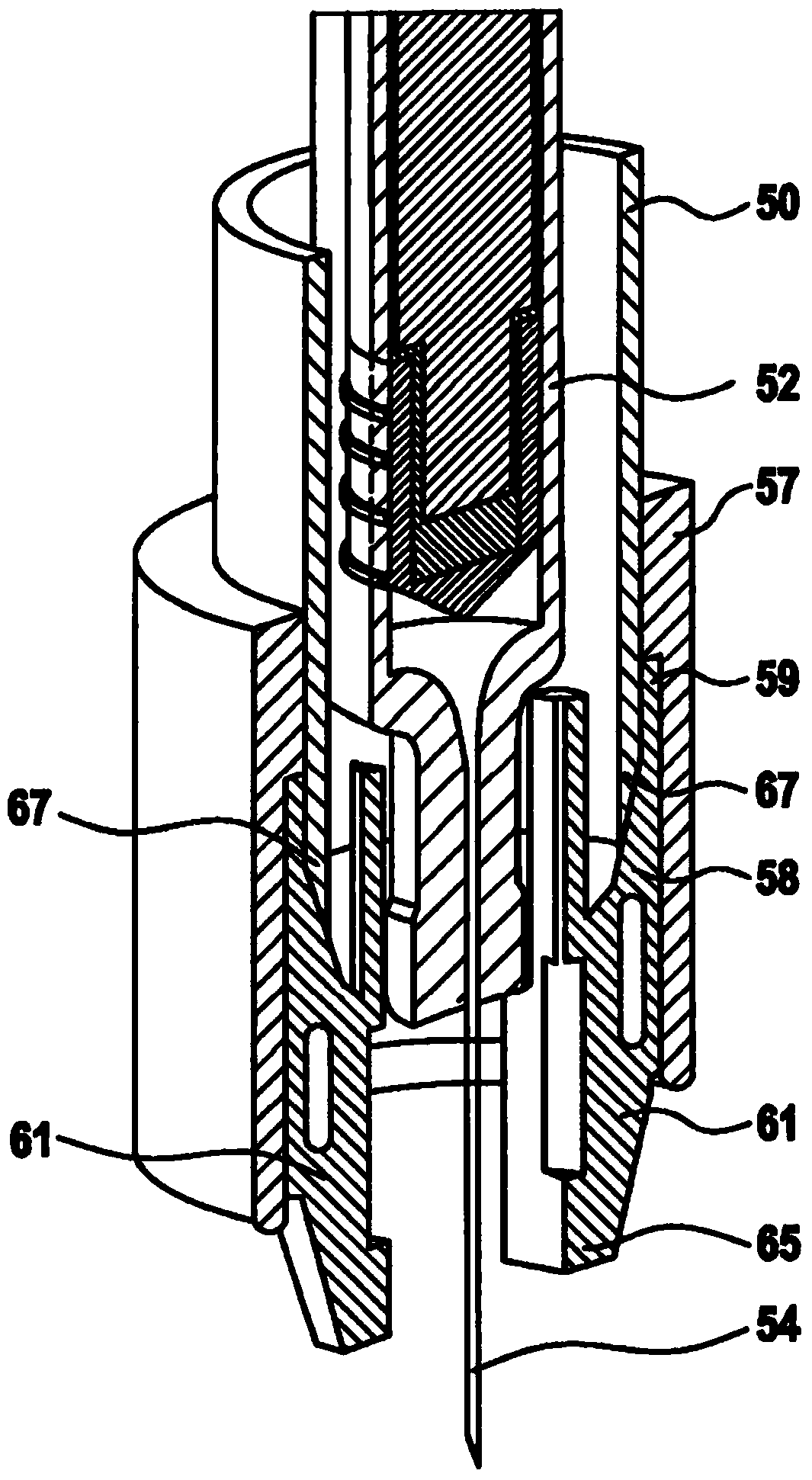
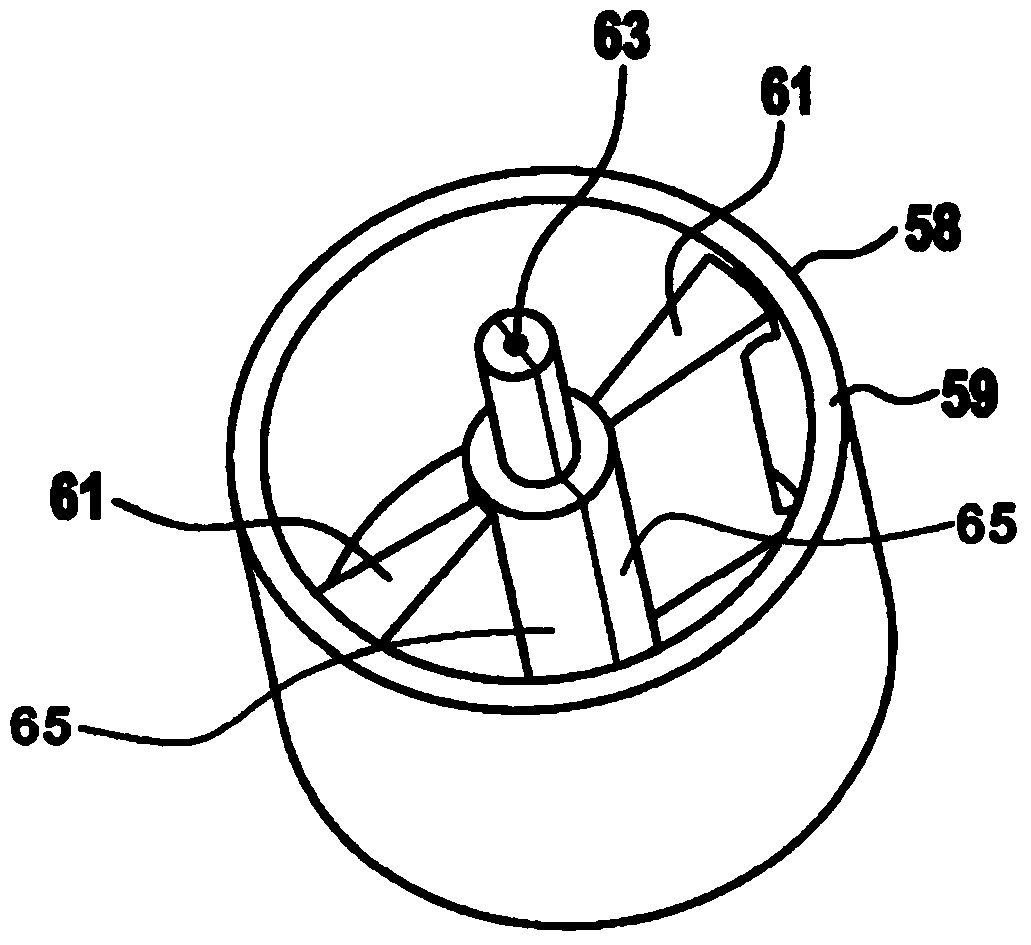
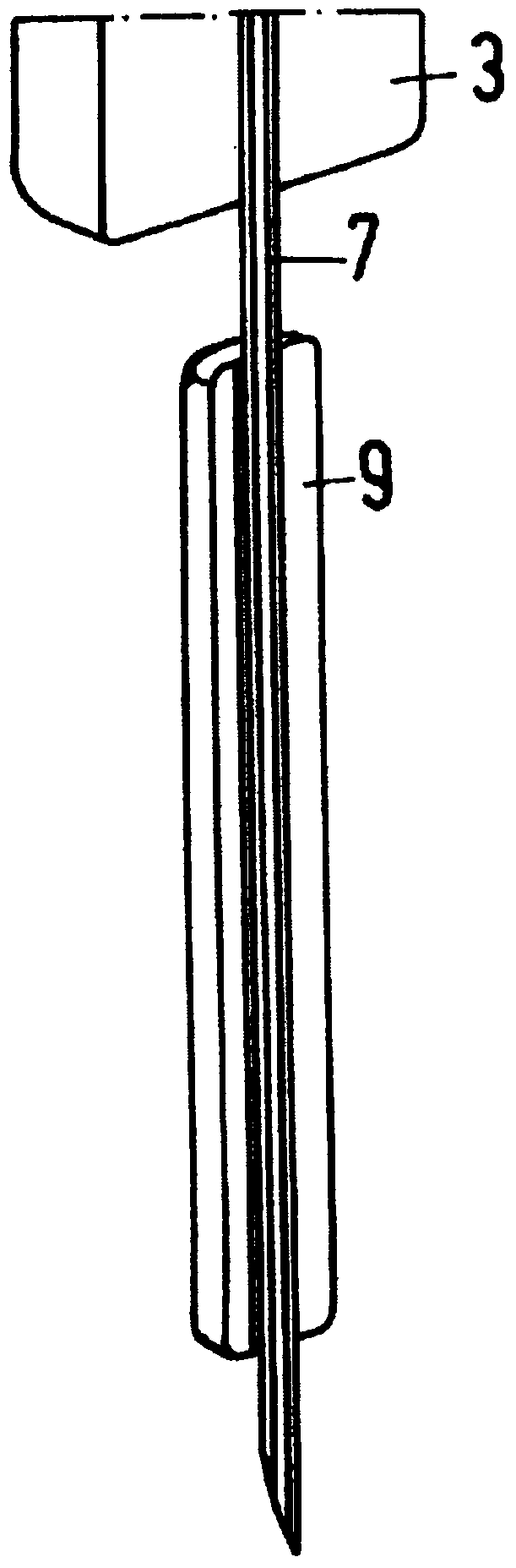
Apparatus for Intraocular Injection
Described is an apparatus for intraocular injection comprising a body (50) adapted to accommodate a syringe (52) having a needle (54), an outer sleeve (57) telescopically coupled to the body (10), and at least two arms (61) hingedly coupled to the outer sleeve (57). Each of the arms (61) comprises a medial portion (65) adapted to encase at least a portion of the needle (54). When the body (50) moves distally relative 10 to said outer sleeve (57), the body (50) engages the arms (61) separating the medial portions (65).
Owner:SANOFI AVENTIS DEUT GMBH
Pharmaceutical formulation
ActiveUS20180235980A1Organic active ingredientsAntipyreticEpidural injectionsPharmaceutical formulation
Disclosed are aqueous pharmaceutical compositions which provide sustained released delivery of corticosteroid compounds. The pharmaceutical composition comprises an insoluble corticosteroid; a soluble corticosteroid; and at least one viscosity enhancing agent. Also provided are methods for using the pharmaceutical compositions in an epidural injection, intra-articular injection, intra-lesional injection, or an intra-ocular injection.
Owner:SEMNUR PHARMA
Method For The Treatment Of Proliferative Disorders Of The Eye
InactiveUS20110200662A1Inhibit cell proliferationLarge therapeutic indexBiocideSenses disorderUveitisMelanoma
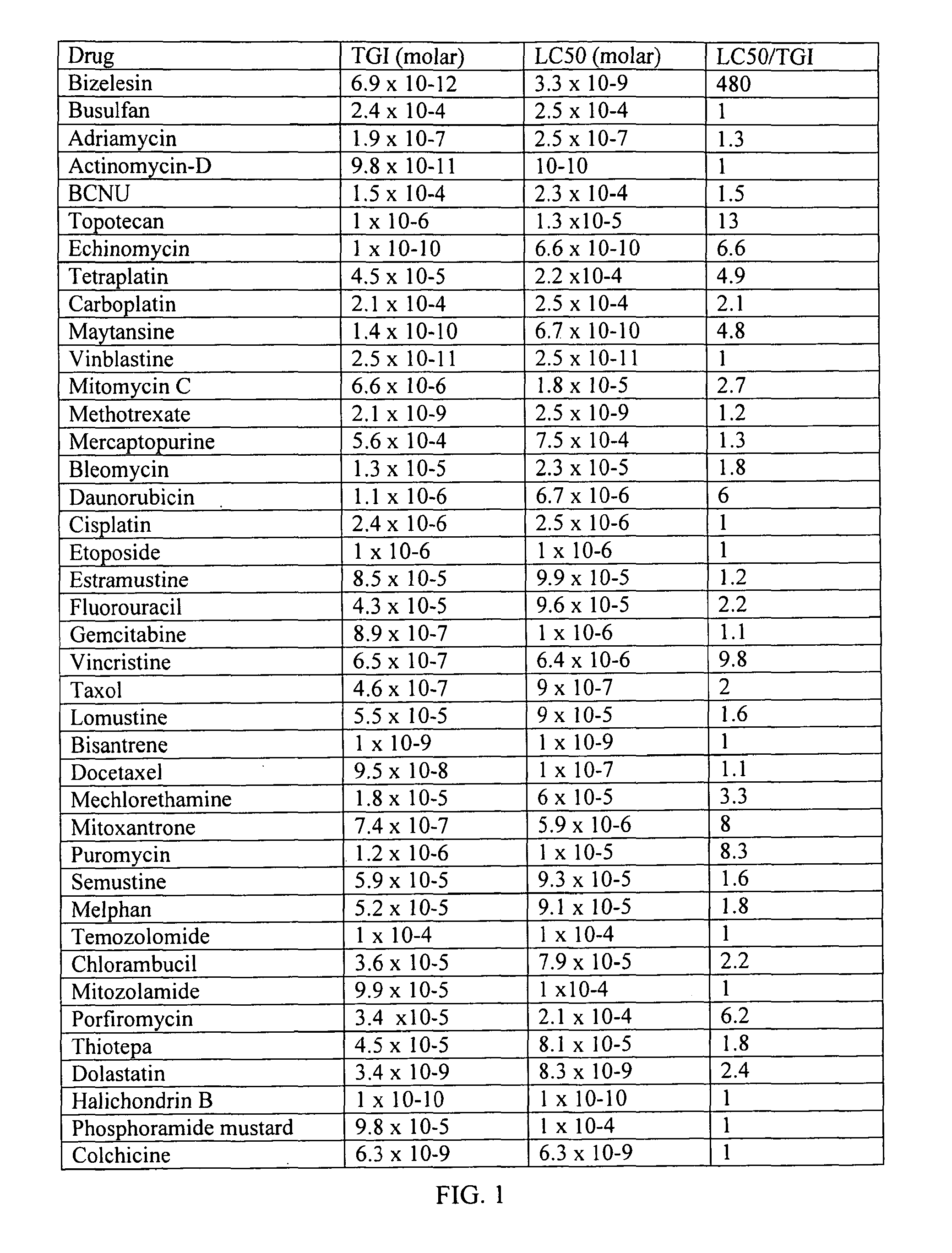
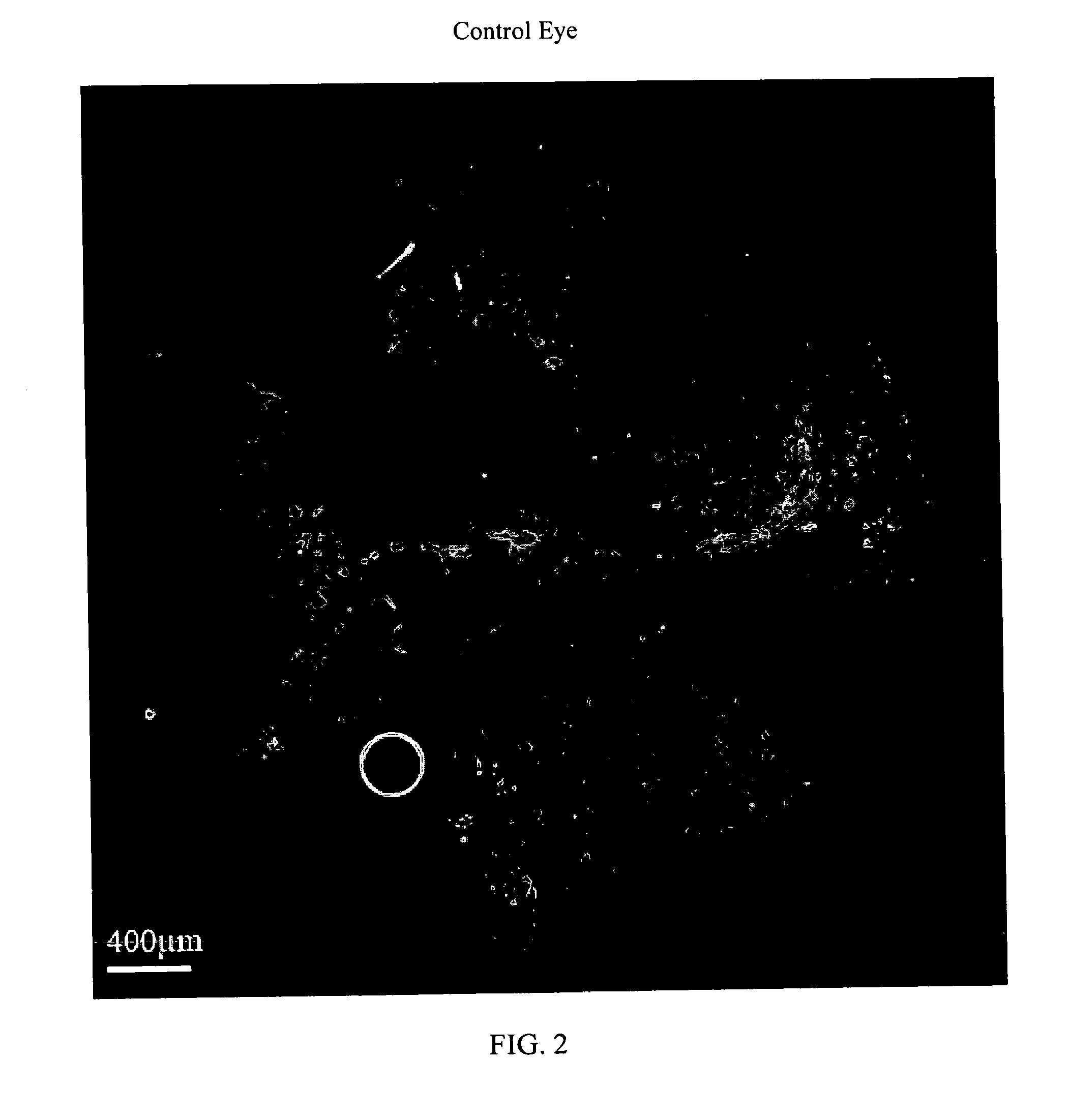
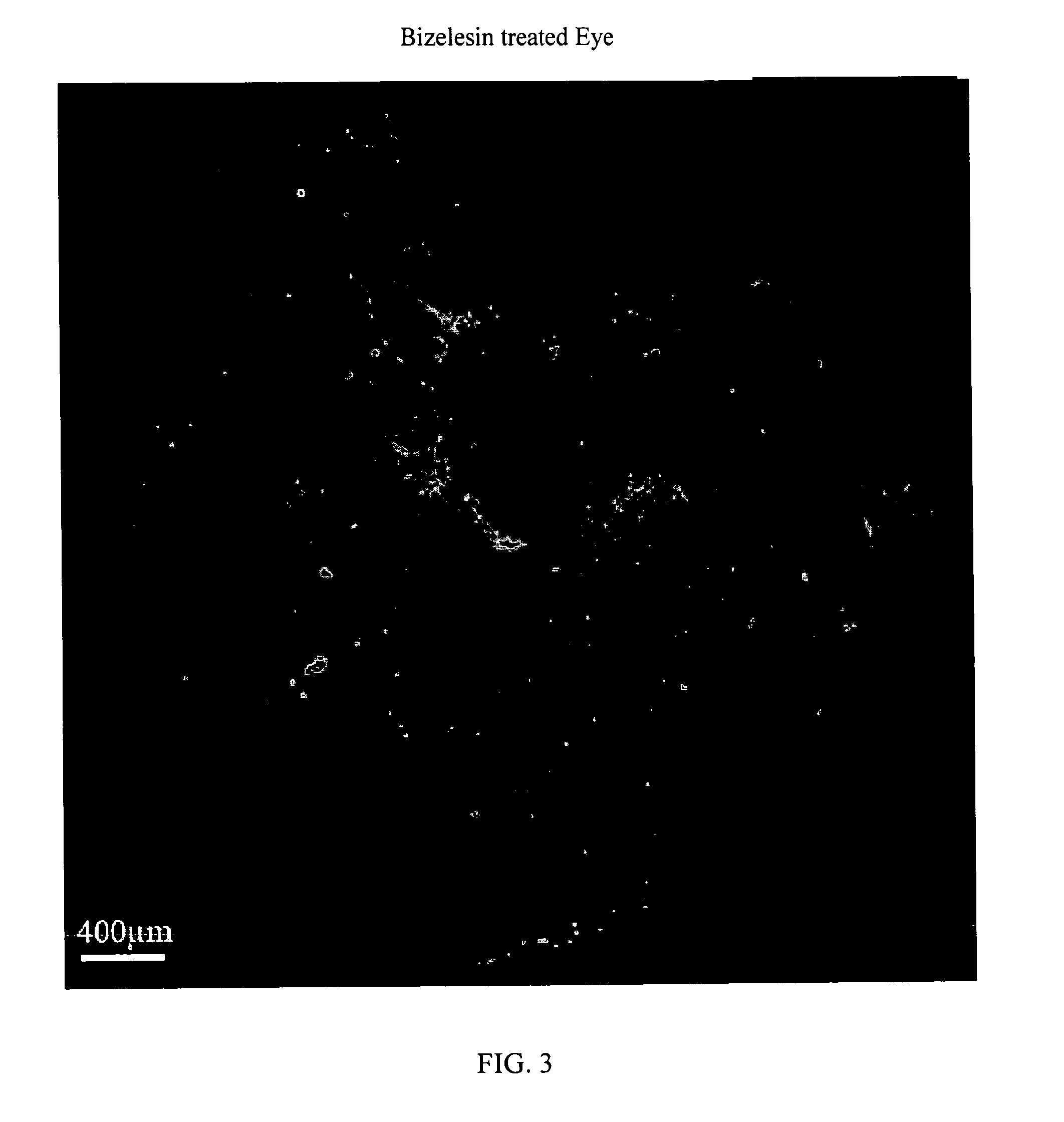
The present invention relates to method for the treatment or prevention and of proliferative eye diseases including but not limited to: age related macular degeneration associated proliferative retinopathy, proliferative diabetic retinopathy, proliferative vitreoretinopathy, posterior capsular opacification, scaring and fibrosis after glaucoma filtration surgery, uveal melanoma, and retinoblastoma. The method comprises contacting cells in the eye by means of intra-ocular injection or infusion, with a drug that irreversibly inhibits cellular proliferation without causing extensive tissue necrosis or cytotoxicity. In a preferred embodiment the drug is bizelesin.
Owner:ONCOTX
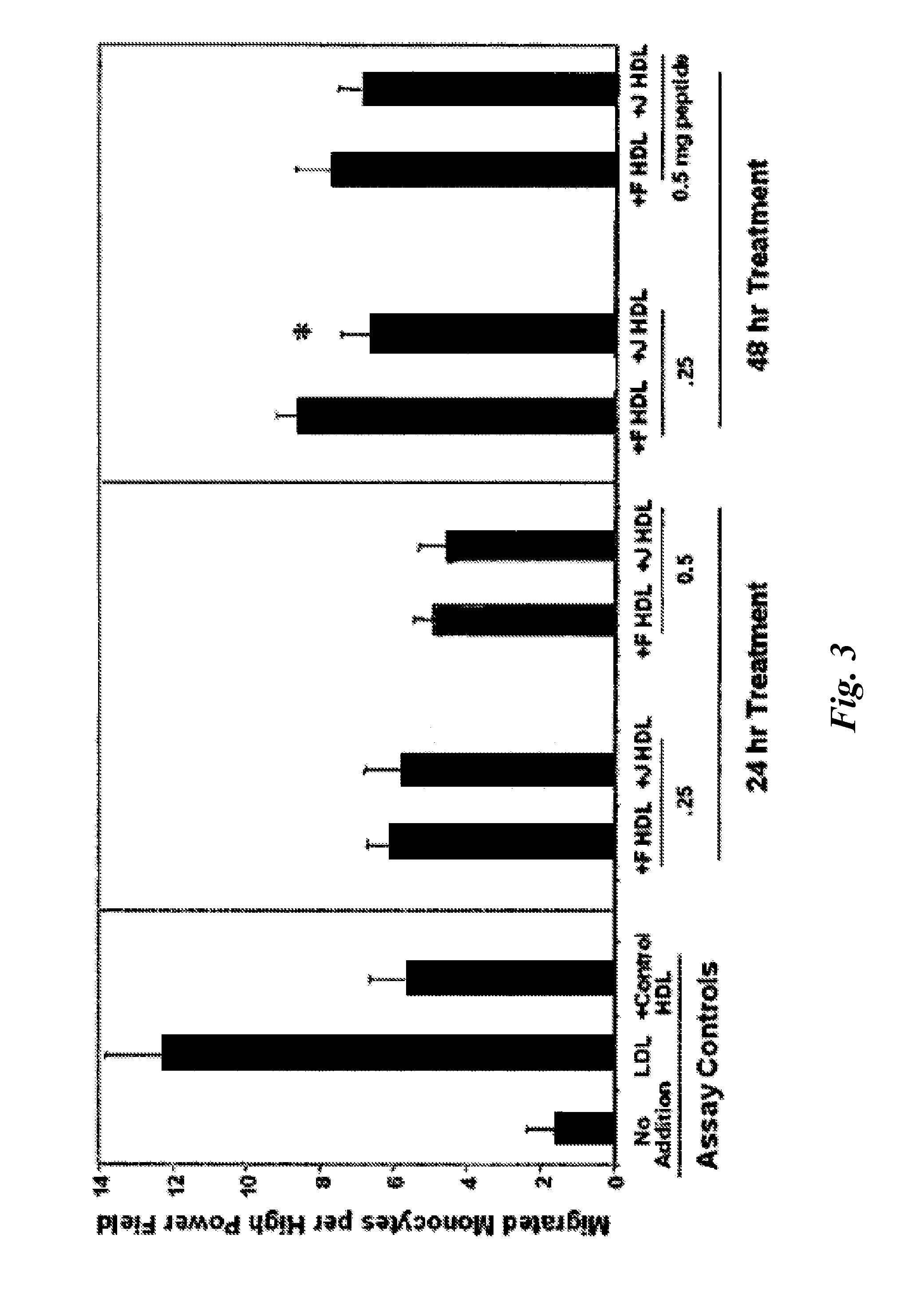
Peptides and peptide mimetics to treat pathologies associated with eye disease
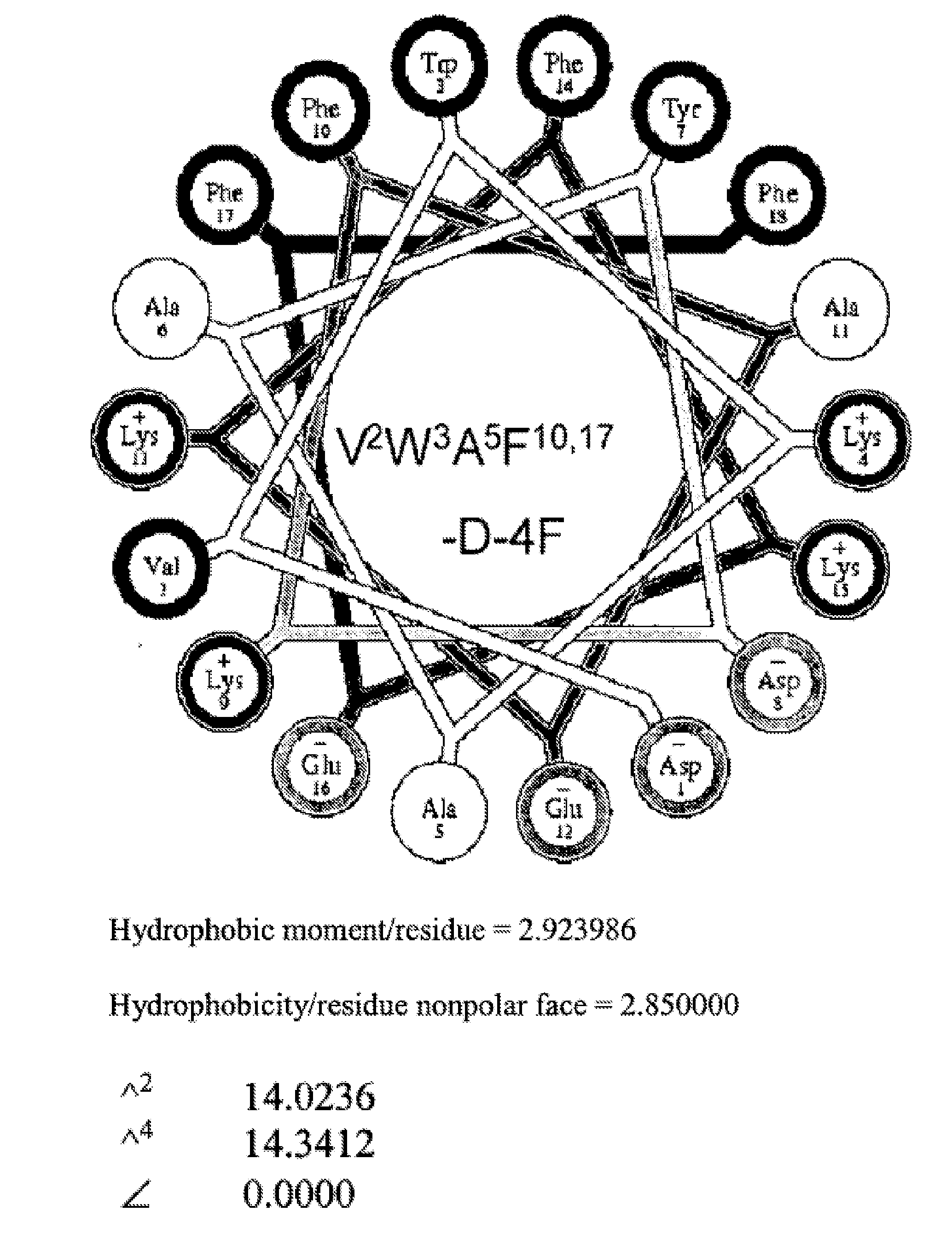
InactiveUS20100143444A1Symptoms improvedSenses disorderPeptide/protein ingredientsAmphipathic helixActive agent
This invention provides novel active agents (e.g. peptides, small organic molecules, amino acid pairs, etc.) peptides that ameliorate one or more symptoms of eye disease and / or other pathologies characterized by an inflammatory response. In certain embodiment, the peptides resemble a G* amphipathic helix of apolipoprotein J. The agents are highly stable and readily administered via an oral route or via intraocular injection.
Owner:RGT UNIV OF CALIFORNIA
Peptides and peptide mimetics to treat pathologies associated with eye disease
This invention provides novel active agents (e.g. peptides, small organic molecules, amino acid pairs, etc.) peptides that ameliorate one or more symptoms of eye disease and / or other pathologies characterized by an inflammatory response, hi certain embodiment, the peptides resemble a G* amphipathic helix of apolipoprotein J. The agents are highly stable and readily administered via an oral route or via intraocular injection.
Owner:UAB RES FOUND
Sterile injectable aqueous formulation used in ophthalmology
InactiveUS20130274224A1Specific spreadingSpecific viscoelasticityBiocideOrganic active ingredientsViscoelasticityHyaluronic acid
The present invention relates to an intraocularly injectable sterile aqueous formulation based on a mixture of hyaluronic acid and alginate, or a salt thereof, used in ophthalmology and having specific viscoelasticity, spreading, covering and ocular tissue adhesion properties and also a high capacity for neutralizing free radicals, said properties enabling said composition to strongly protect the eye tissues.
Owner:ANTEIS SA
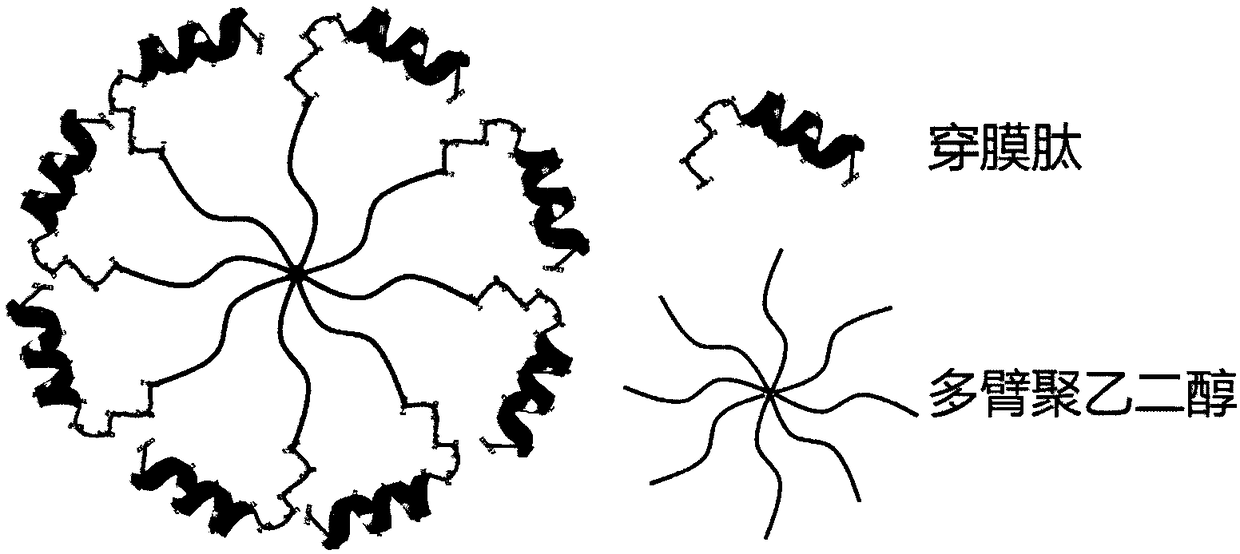
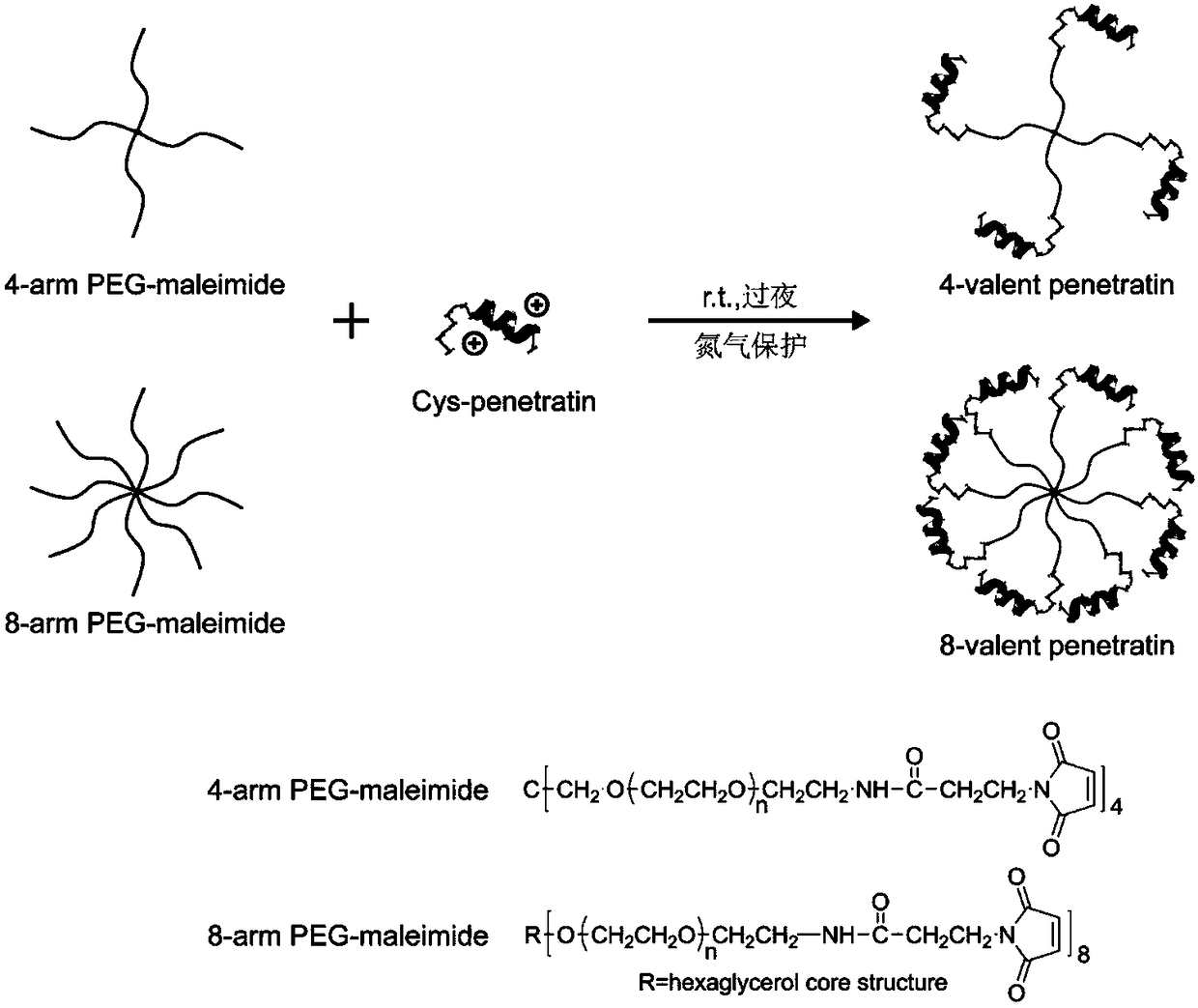
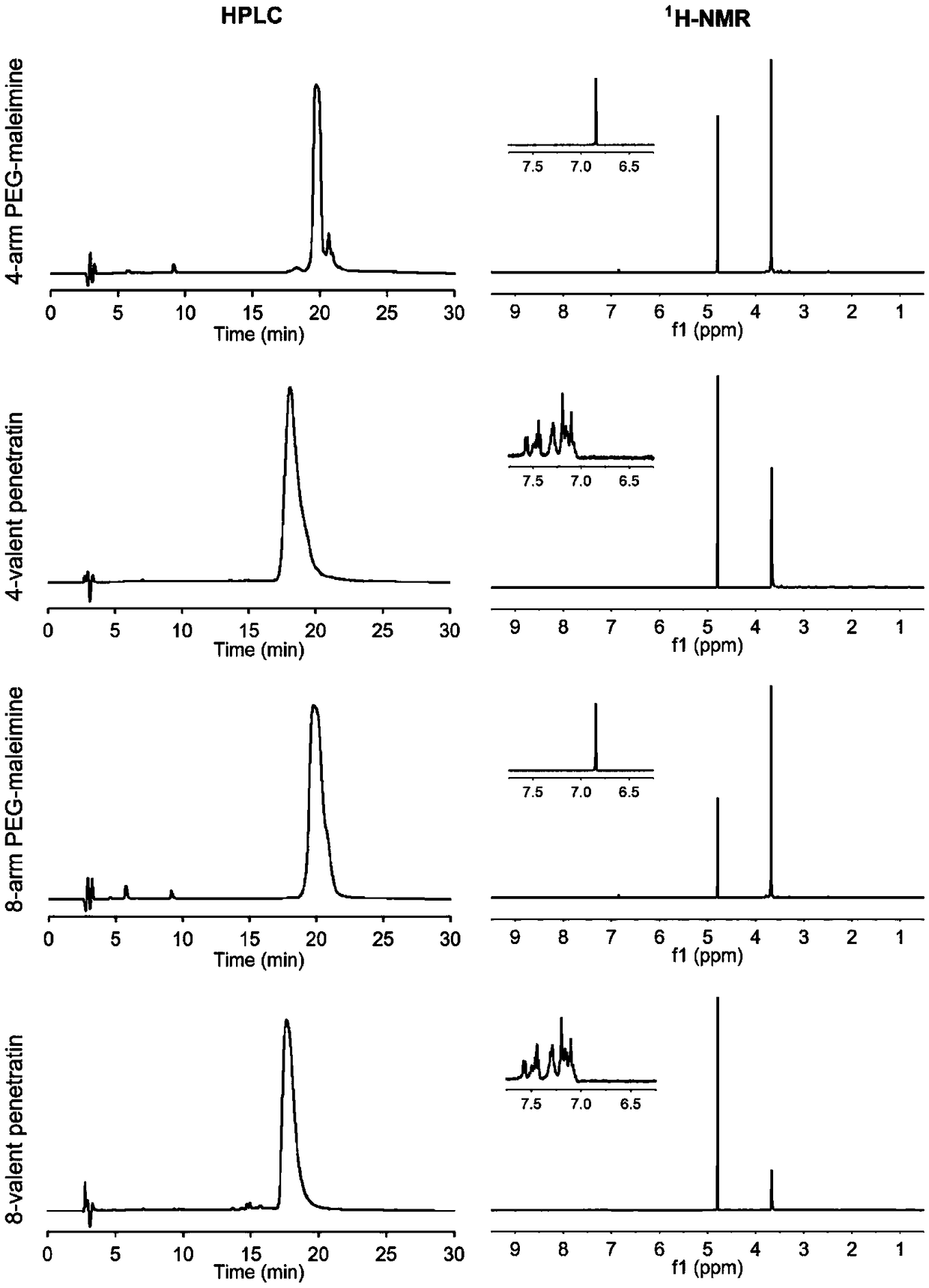
Multivalent cell-penetrating peptide biological macromolecule delivery vector and application thereof
PendingCN109420178AReduce deliveryIncrease intakeSenses disorderPeptide/protein ingredientsDiseasePoor compliance
The invention belongs to the field of pharmaceutical preparations, and relates to a series multivalent cell-penetrating peptide delivery vector and a complex thereof, wherein the vector is built by the aid of multi-arm polyethylene glycol and cell-penetrating peptides or cell-penetrating peptide derivatives. The multivalent cell-penetrating peptide delivery vector has an octopus-like flexible structure, can be self-assembled with biological macromolecules, particularly genes to form the non-covalent complex and has strong biological macromolecule carrying, biological macromolecule delivery andocular tissue penetrating capacity, ocular tissue toxicity is avoided, biological macromolecule drugs can be effectively delivered to an intraocular portion or ocular fundus in a noninvasive way, andbiological macromolecule drug uptake by ocular tissues is increased. The complex formed by self-assembling the multivalent cell-penetrating peptide delivery vector with the biological macromoleculescan be used for eye drop administration, intraocular injection administration with poor compliance to patients can be replaced, and intraocular and ocular fundus disease treatment convenience and safety are enhanced.
Owner:FUDAN UNIV
Application of pyrryl-substituted indole compound in curing glaucoma
ActiveCN103127096ARelieve symptomsReduce blinding rateOrganic active ingredientsSenses disorderMedicineEye drop
Owner:杨子娇
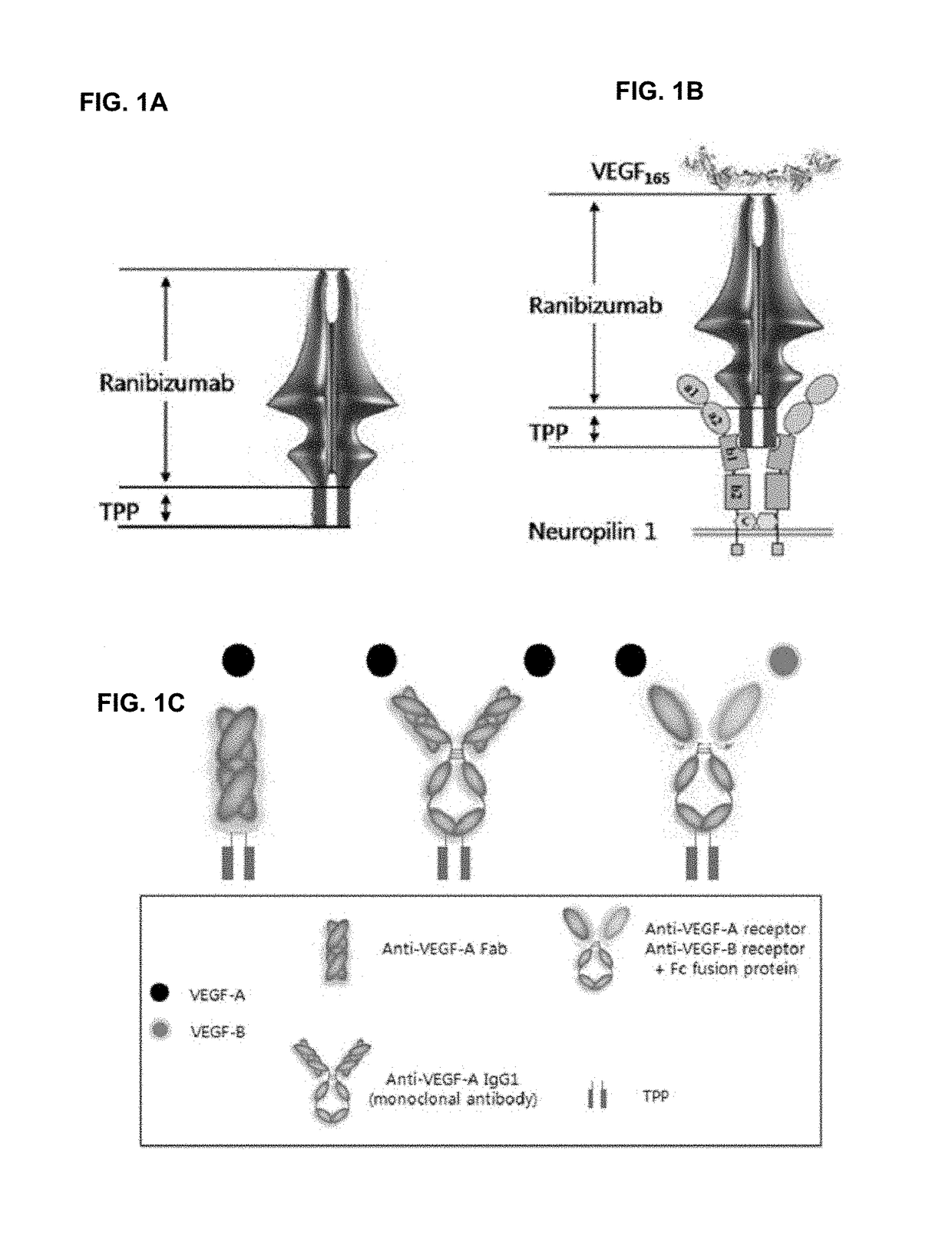
Pharmaceutical composition for preventing and treating eye diseases, containing, as active ingredient, fusion protein in which tissue-penetrating peptide and Anti-vascular endothelial growth factor preparation are fused
ActiveUS20180133288A1Improved tissue penetrationImprove abilitiesSenses disorderPeptide/protein ingredientsDiseaseFactor ii
The present invention relates to a pharmaceutical composition for preventing and treating eye diseases, comprising, as an active ingredient, a fusion protein in which a tissue-penetrating peptide and an anti-vascular endothelial growth factor (anti-VEGF) preparation are fused. More particularly, the present invention relates to: a pharmaceutical composition for preventing and treating eye diseases, comprising, as an active ingredient, a fusion protein in which a tissue-penetrating peptide and an anti-VEGF preparation are fused; a method for prepraring an anti-VEGF preparation, which overcomes resistance and has an improved efficacy, the method comprising the steps of transforming a host cell with a recombinant vector comprising a nucleic acid sequence encoding a fusion protein in which a tissue-penetrating peptide and an anti-VEGF preparation are fused, culturing the cell, and recovering a fusion protein from the cell; a method for treating eye diseases, comprising administering an effective dose of the fusion protein according to the present invention to a subject in need thereof; and use of the fusion protein according to the present invention for preparing an agent for treating eye diseases. Compared to conventional anti-VEGF preparations, the composition according to the present invention is considered to have an improved efficacy and be able to be used for treating patients having drug resistance, by inhibiting various growth factors related to new blood vessels, besides VEGF, and by decreasing pericyte coverage. In addition, since drug delivery ability into the choroid tissue is improved when performing an intraocular injection, the composition can be developed as eye drops by reducing an administered dosage or extending an administration cycle, and by improving ocular penetrability.
Owner:IL DONG PHARMA CO LTD
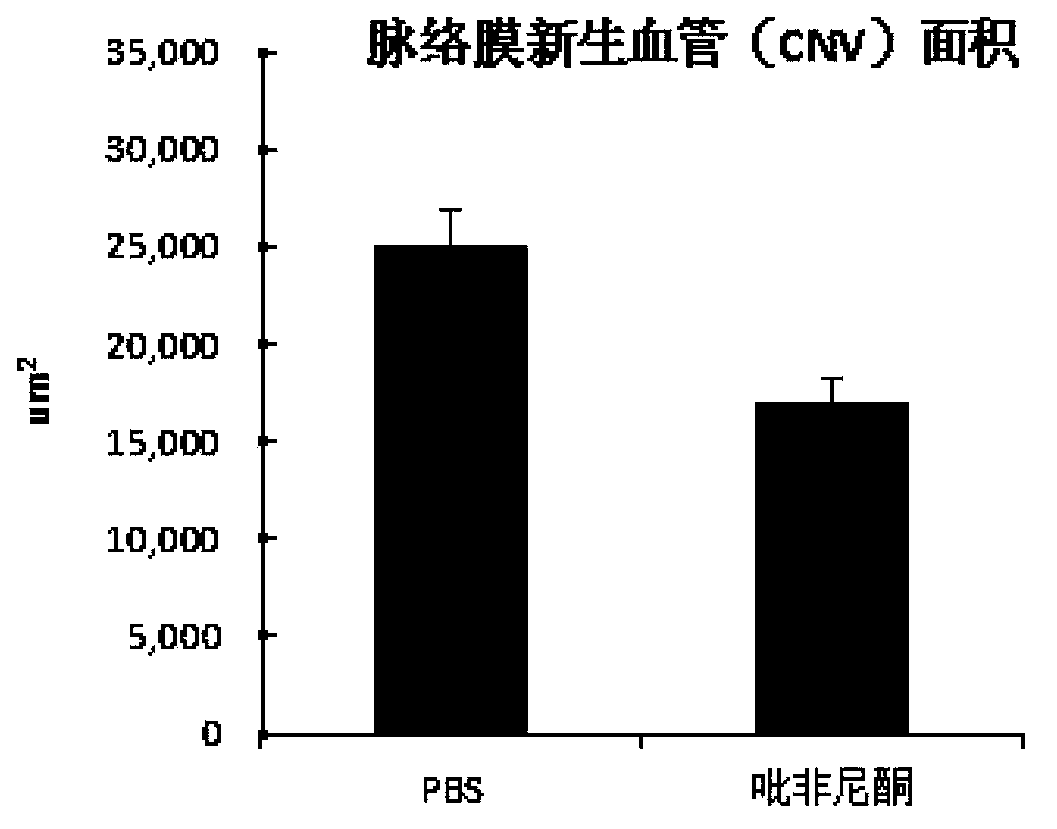
Novel application of pirfenidone
InactiveCN103800325AInhibition of newbornsGood treatment effectOrganic active ingredientsSenses disorderDiseaseTreatment effect
The invention provides application of pirfenidone in preparing anti-angiogenesis medicines, and provides a medicine for treating wet macular degeneration. Pirfenidone can be used for effectively inhibiting choroid angiogenesis, has an excellent activity by eye dropping and intraocular injection, can be used for treatment of angiogenesis diseases in ophthalmological department, in particular remarkable treatment effect to wet macular degeneration, and provides a novel choice for clinical administration.
Owner:张康
Intraocular injection for treating infectious endophthalmitis
InactiveCN101455836AGood treatment effectNo side effectsAntibacterial agentsOrganic active ingredientsSide effectInfectious endophthalmitis
The invention relates to an intraocular injection for treating infectious endophthalmitis, which is a drug for treating the infectious endophthalmitis. According to clinical studies and animal experiments, because of the incorrect selection of drugs, drug concentration and safe and effective doses, the toxicity causes damages to retina. 1.5 percent norvancomycin hHydrochloride and 0.5 percent dexamethasone sodium phosphate are mixed together according to the mass ratio of 3:1, thereby obtaining specific effects on the treatment of endophthalmitis patients. The intraocular injection has the advantages of significant curative effect, no toxicity, no side effects, and the like.
Owner:戴成华
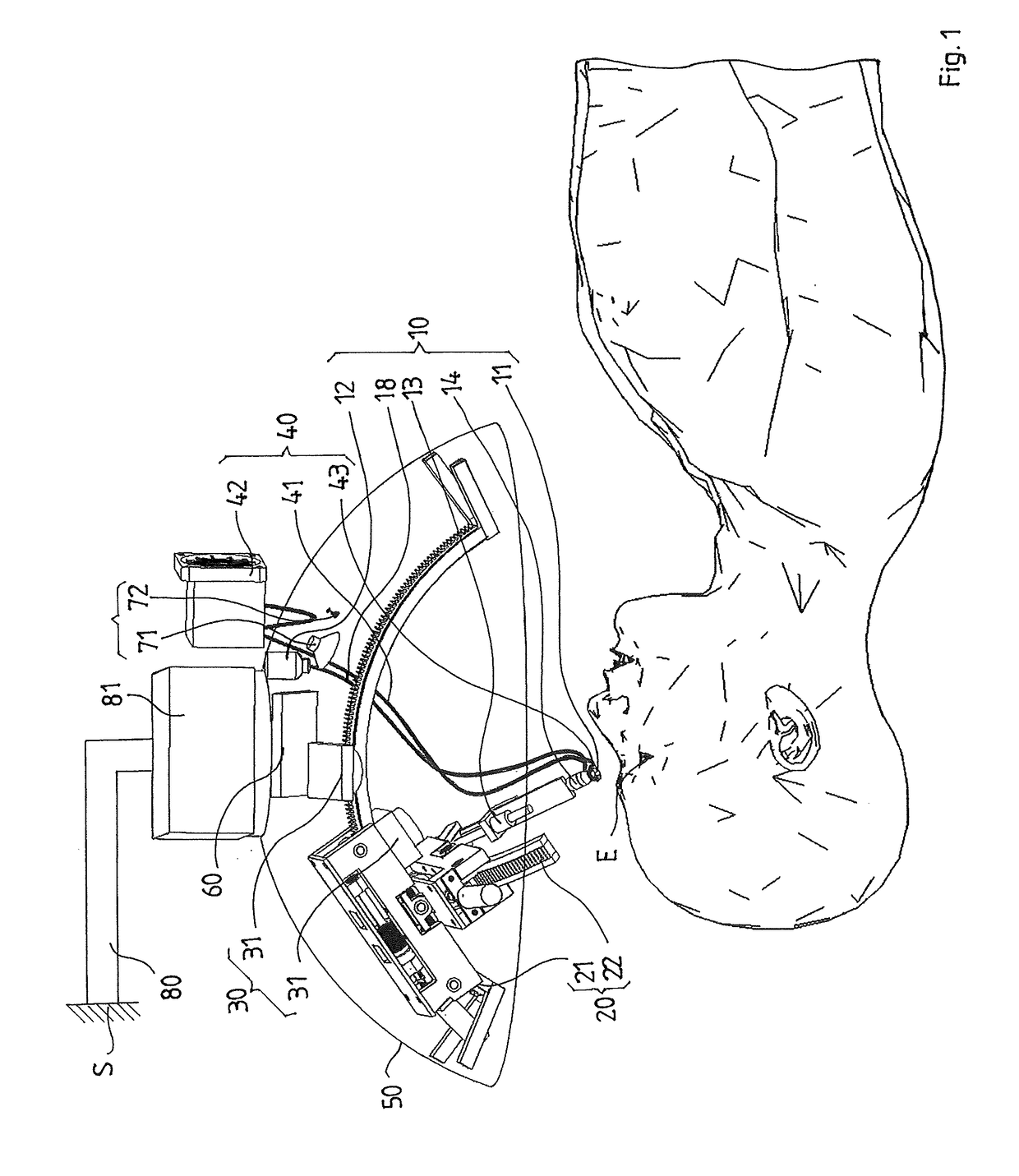
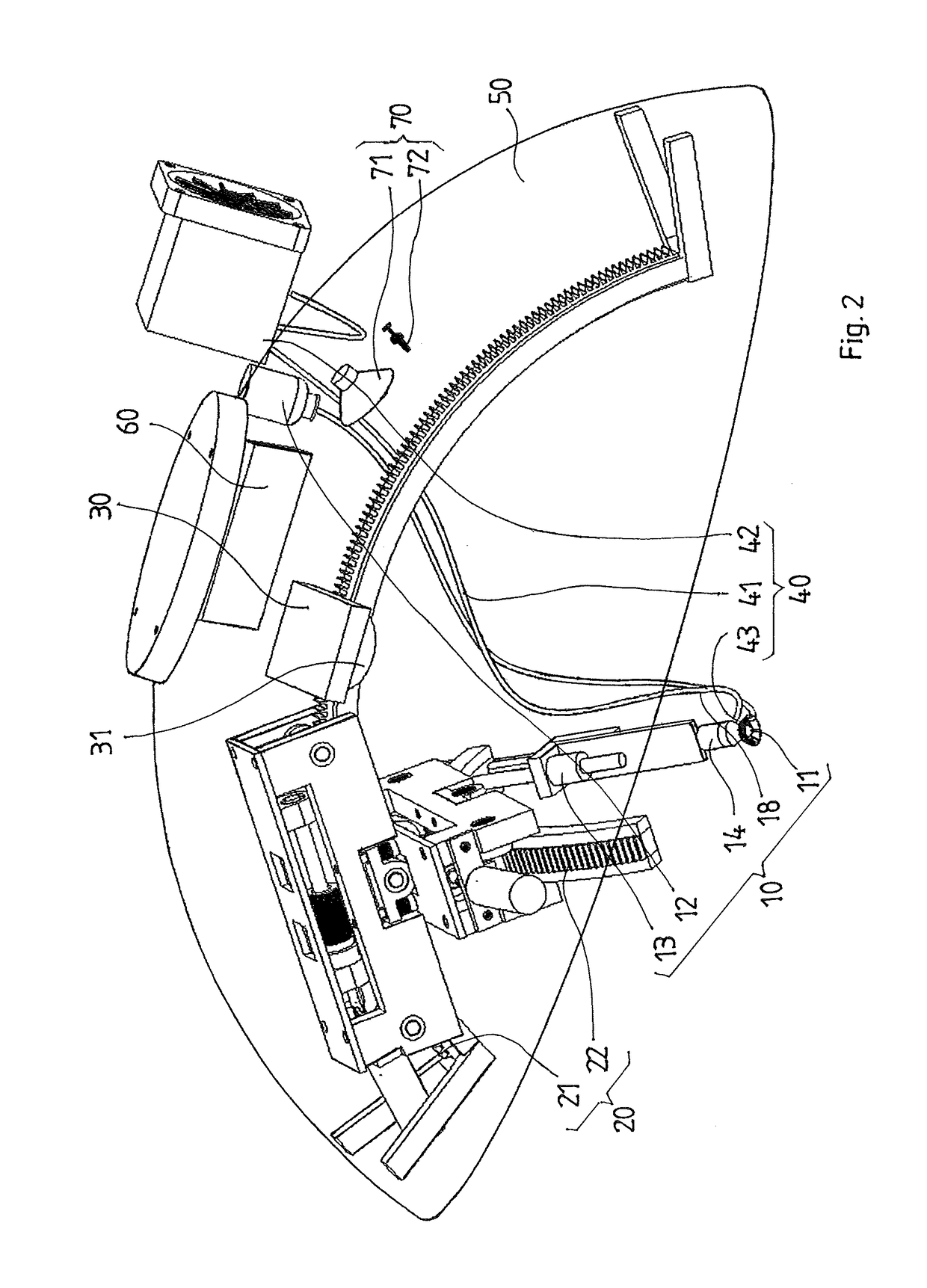
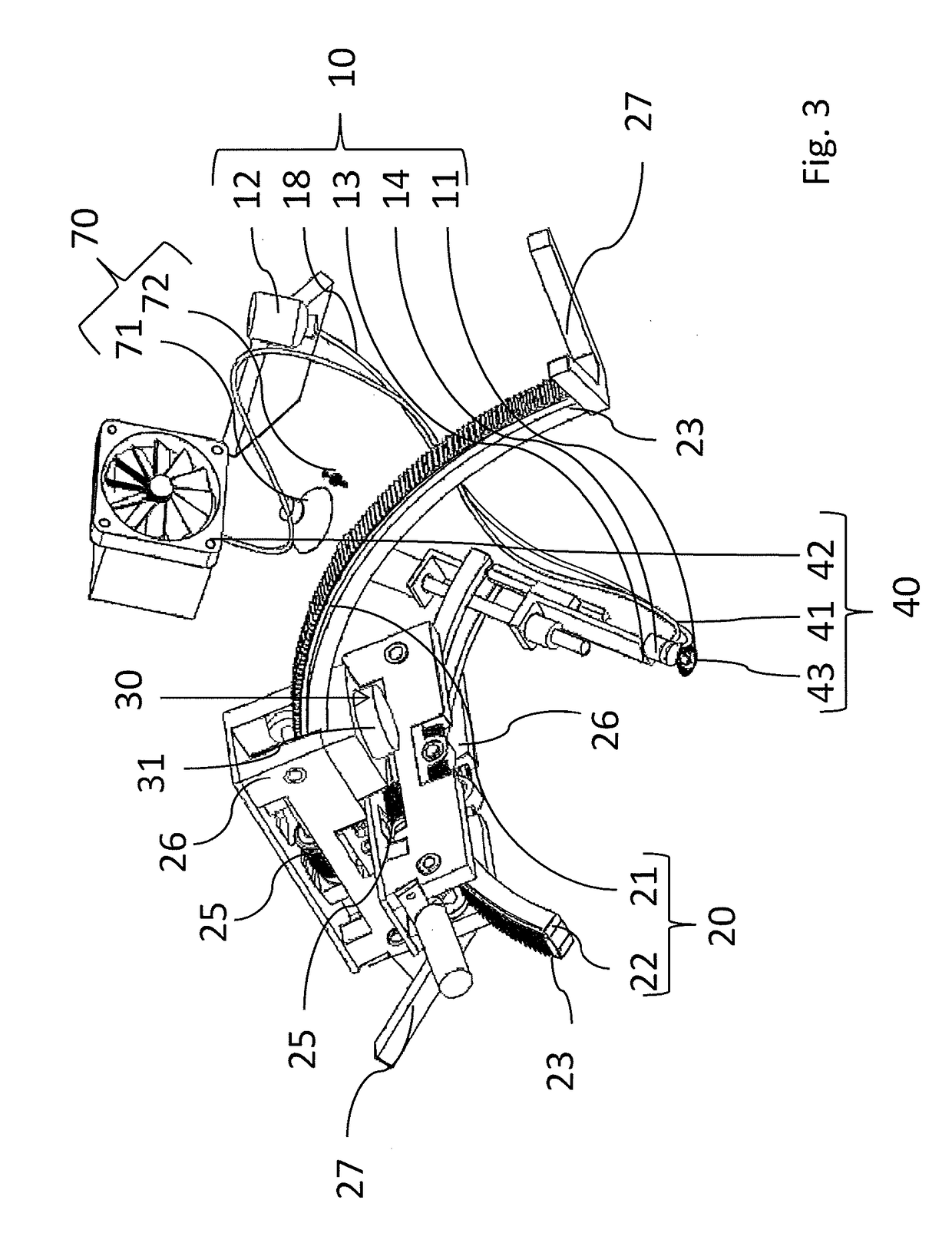
Intraocular injection system and methods for controlling such a system
Owner:OPHTHOROBOTICS AG
Anti-virus sustained-release drug capable of being subjected to intraocular injection, preparation method and applications thereof
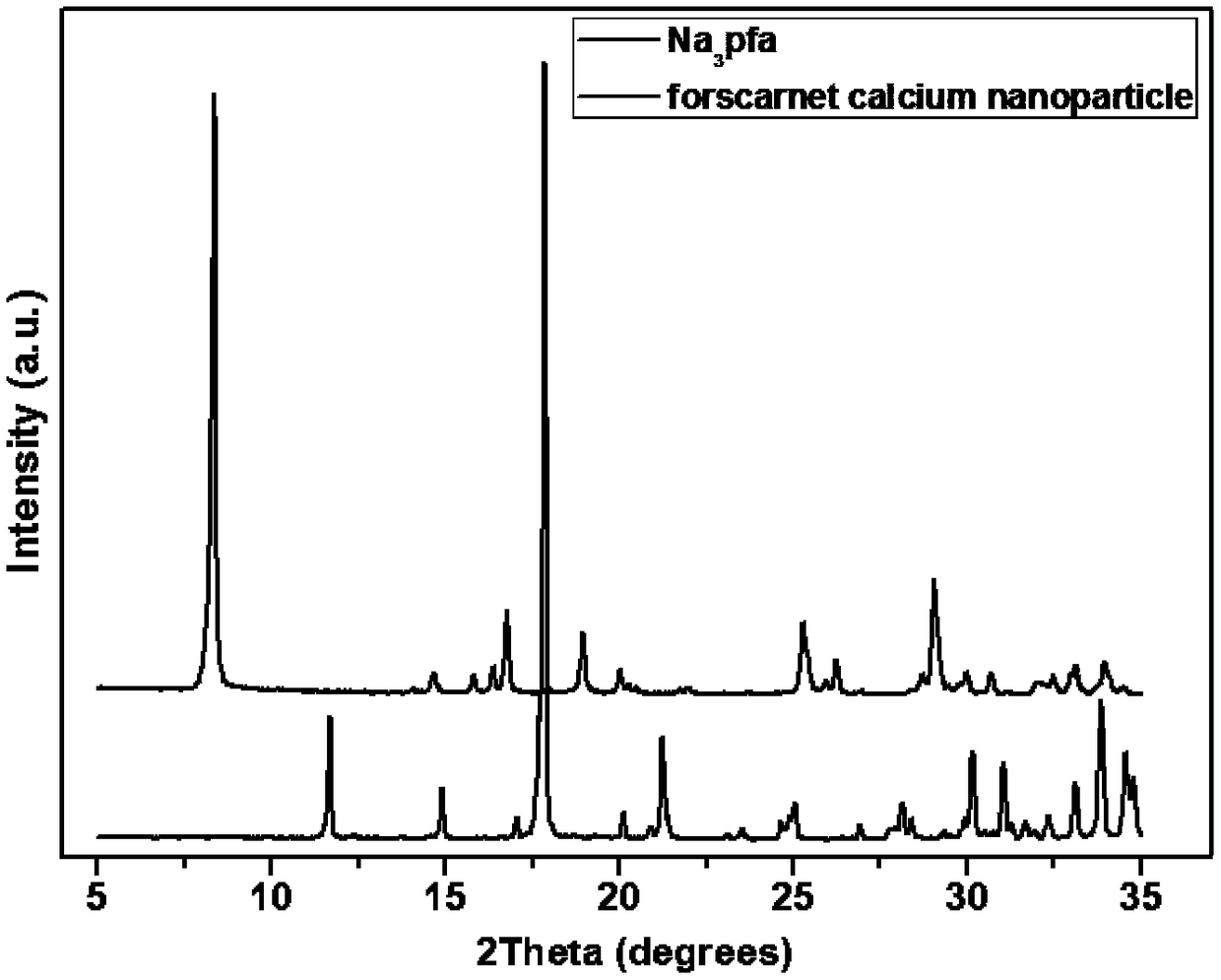
ActiveCN108276440AGood for healthSlow release and long-lasting stabilityOrganic active ingredientsSenses disorderAnti virusFoscarnet
The invention provides an anti-virus sustained-release drug capable of being subjected to intraocular injection, a preparation method and applications thereof, wherein the anti-virus sustained-releasedrug is a foscarnet insoluble salt micro-crystal, and the foscarnet insoluble salt is the polyvalent metal salt of foscarnet. The preparation method comprises: carrying out a reaction on the monovalent metal salt of foscarnet and the soluble salt of a polyvalent metal in water to obtain the system containing the foscarnet insoluble salt micro-crystal. According to the present invention, based onthe special requirement of the controlled-release and slow-release intraocular injection, the foscarnet insoluble salt micro-crystal slow-release system is designed, wherein the nominal drug loading can be more than 100%; the foscarnet insoluble salt micro-crystal slow-release drug can be lastingly, stably and slowly released in the vitreous cavity of rabbit eyes, the concentration of the foscarnet in the vitreous cavity is always maintained above the effective therapeutic concentration of sodium foscarnet, the sustained release period is up to 12 weeks, and the drug does not have obvious toxic effects and inflammatory reaction; and the foscarnet insoluble salt can be used as the anti-virus sustained-release drug capable of being injected into the vitreous cavity.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV +1
Aid device for intraocular injection
Owner:SANOFI AVENTIS DEUT GMBH
Intraocular injection device
Described is a medicament delivery device (1) comprising a needle (7) and a layer (9) of a composition coupled to at least a portion of the needle (7). The composition has a low viscosity and a low surface tension.
Owner:SANOFI AVENTIS DEUT GMBH
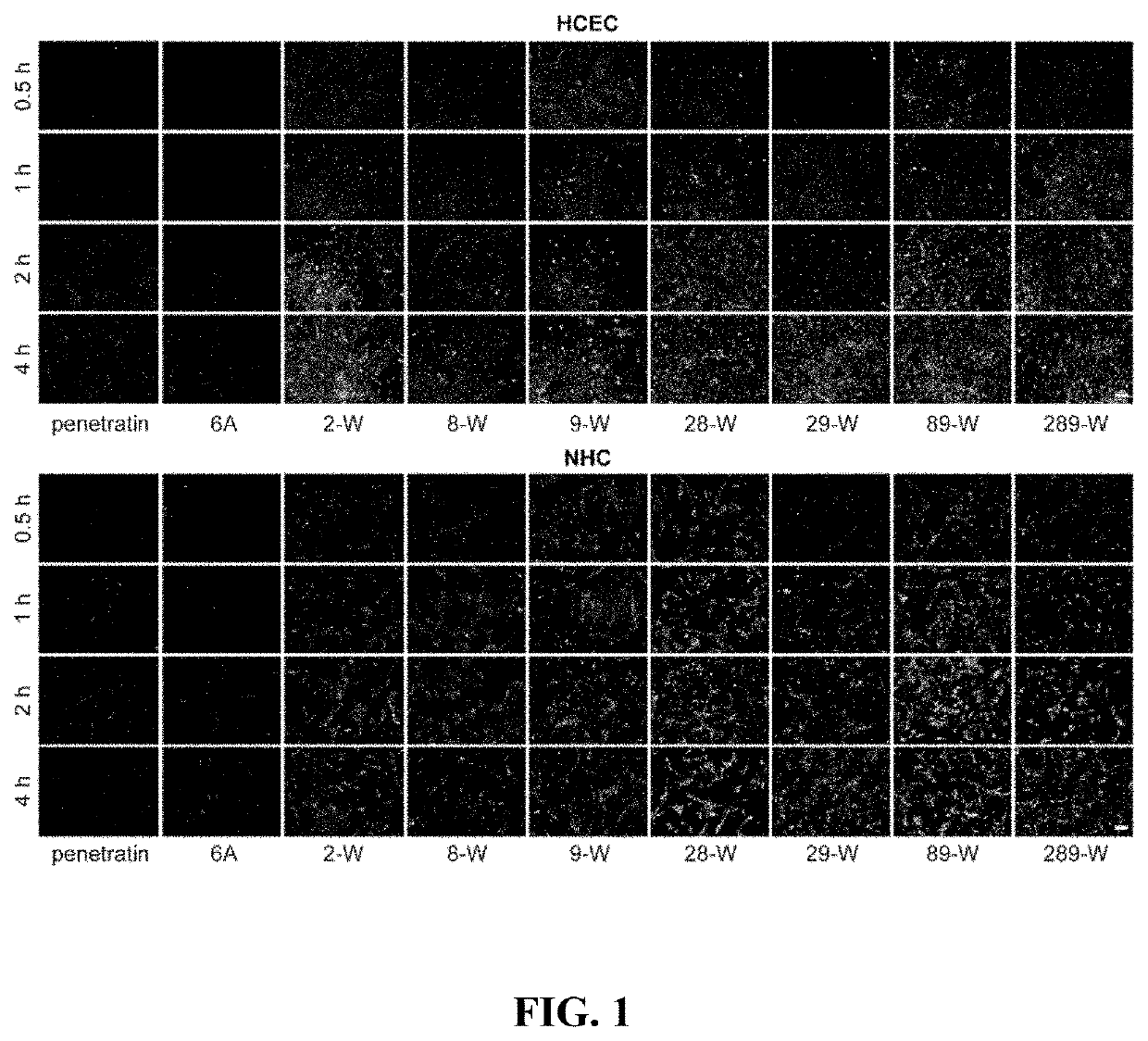
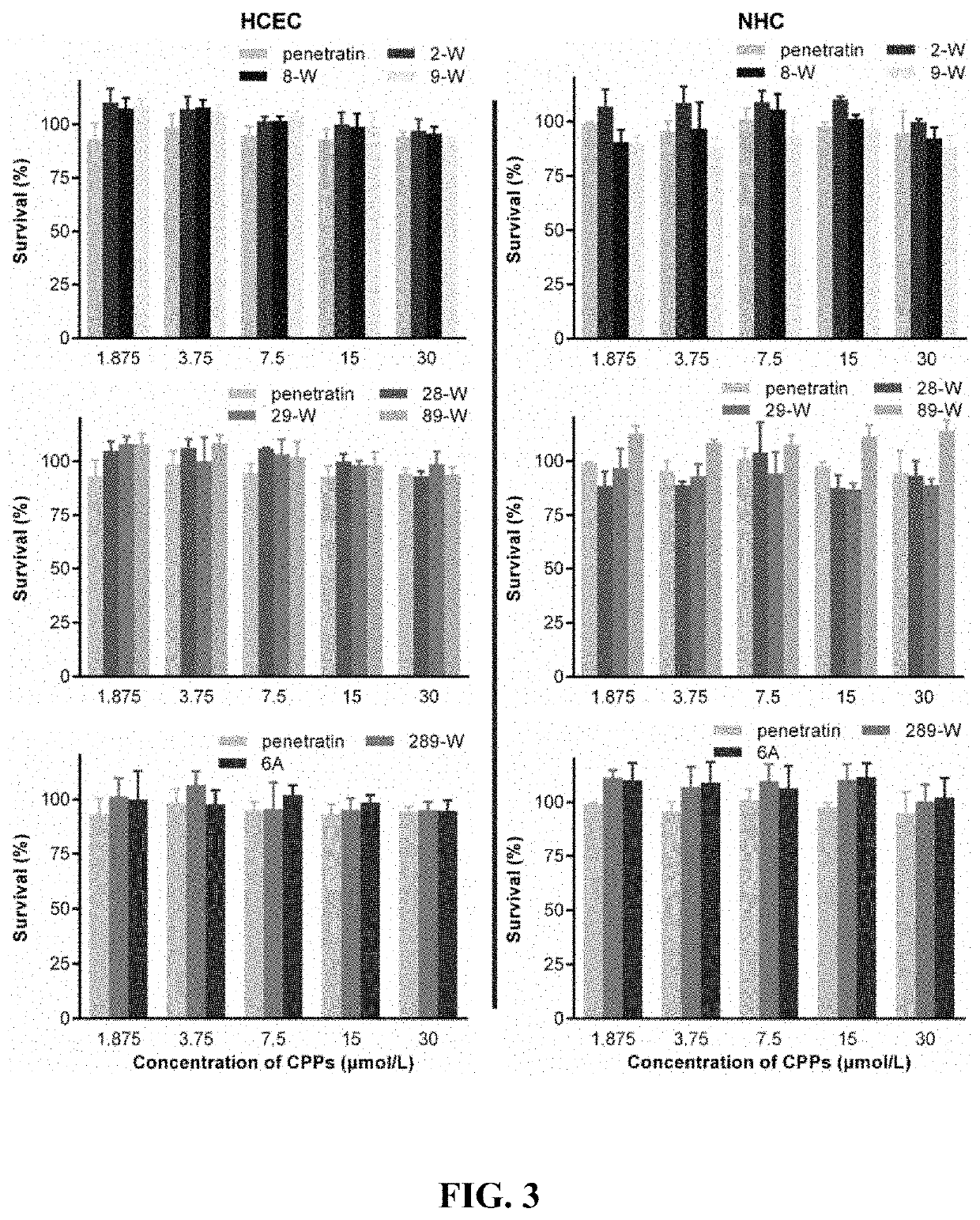
Polypeptide eye absorption enhancer and use thereof
ActiveUS11213591B2Short stayImprove bioavailabilityAntibacterial agentsSenses disorderOcular bioavailabilityDisease
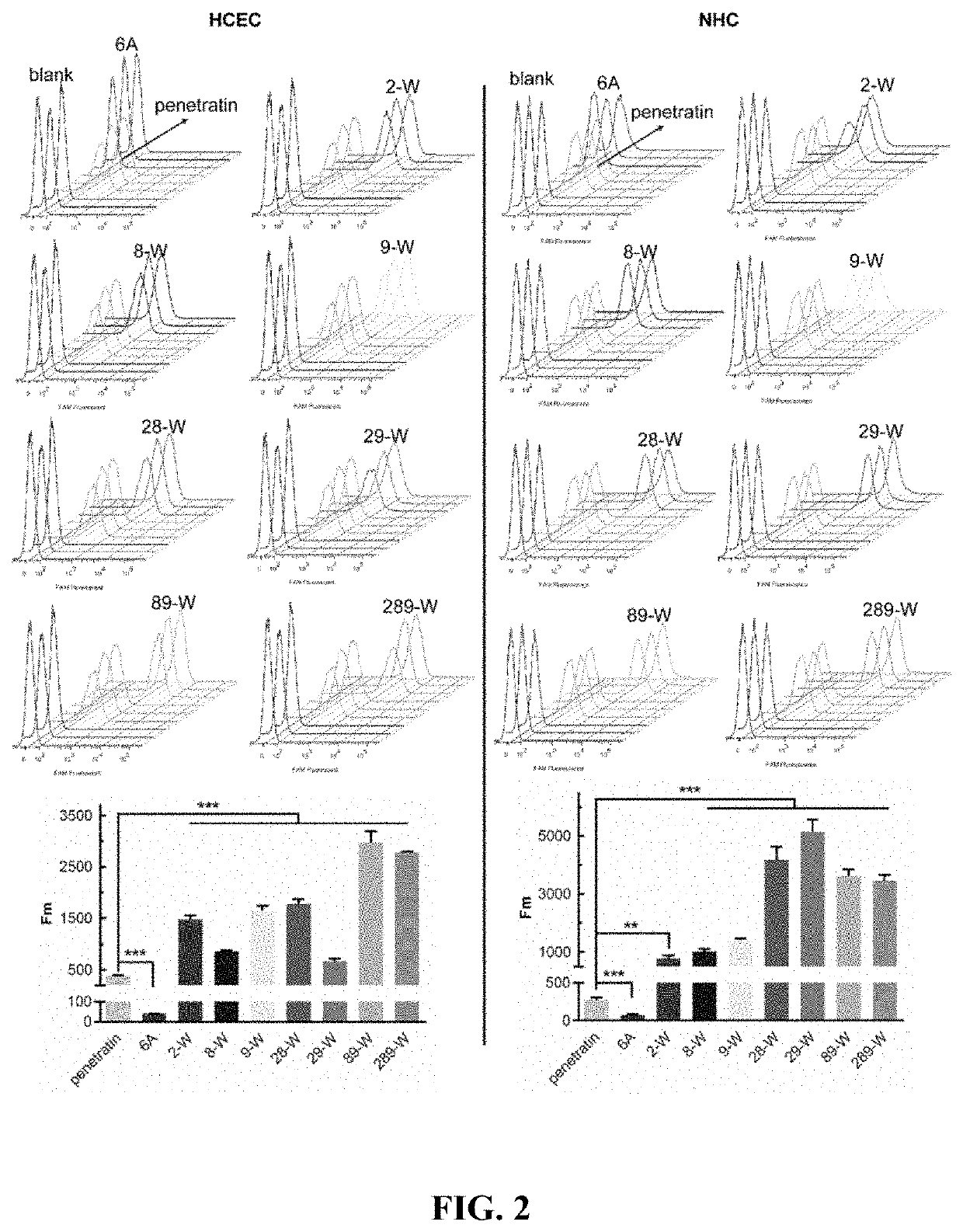
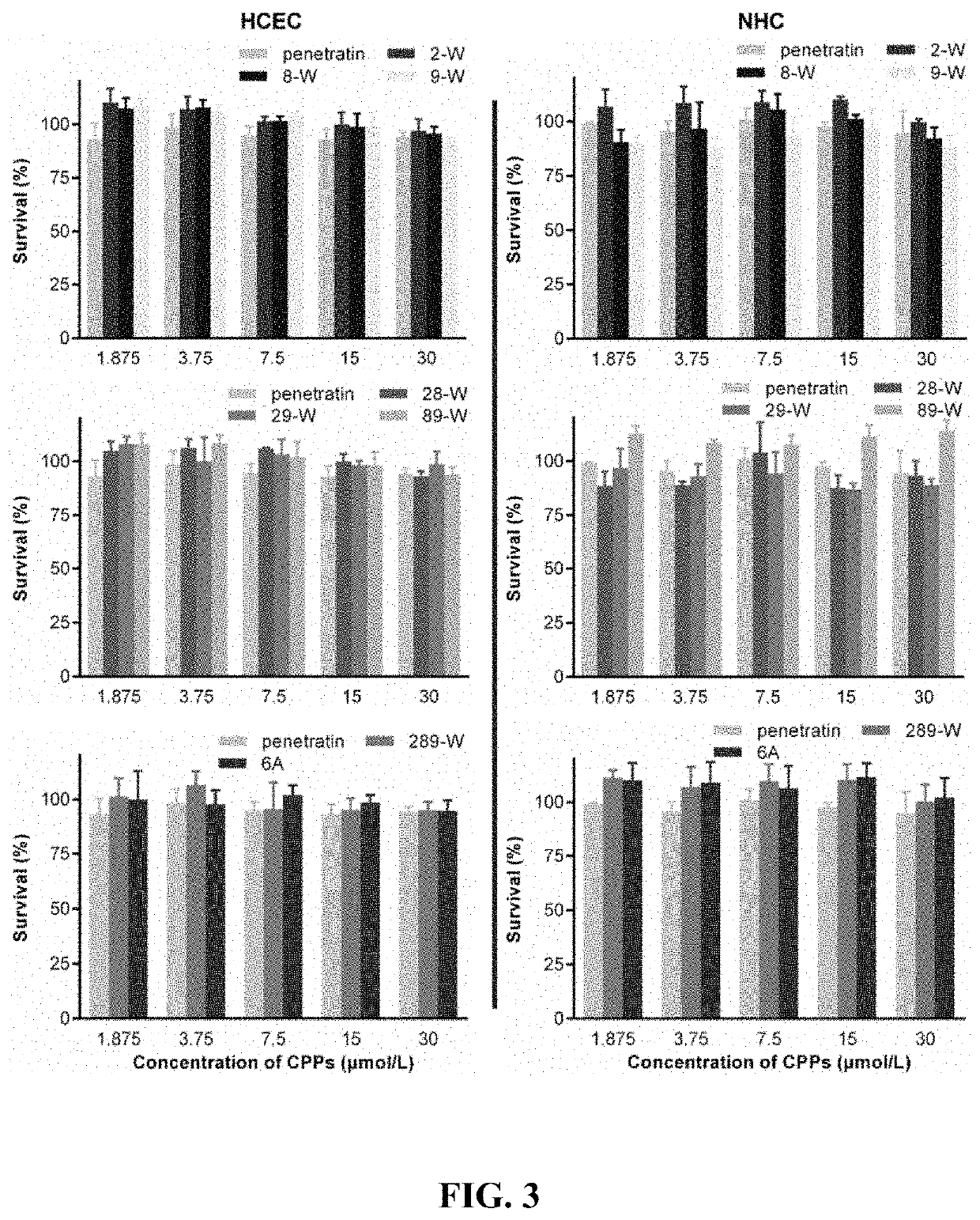
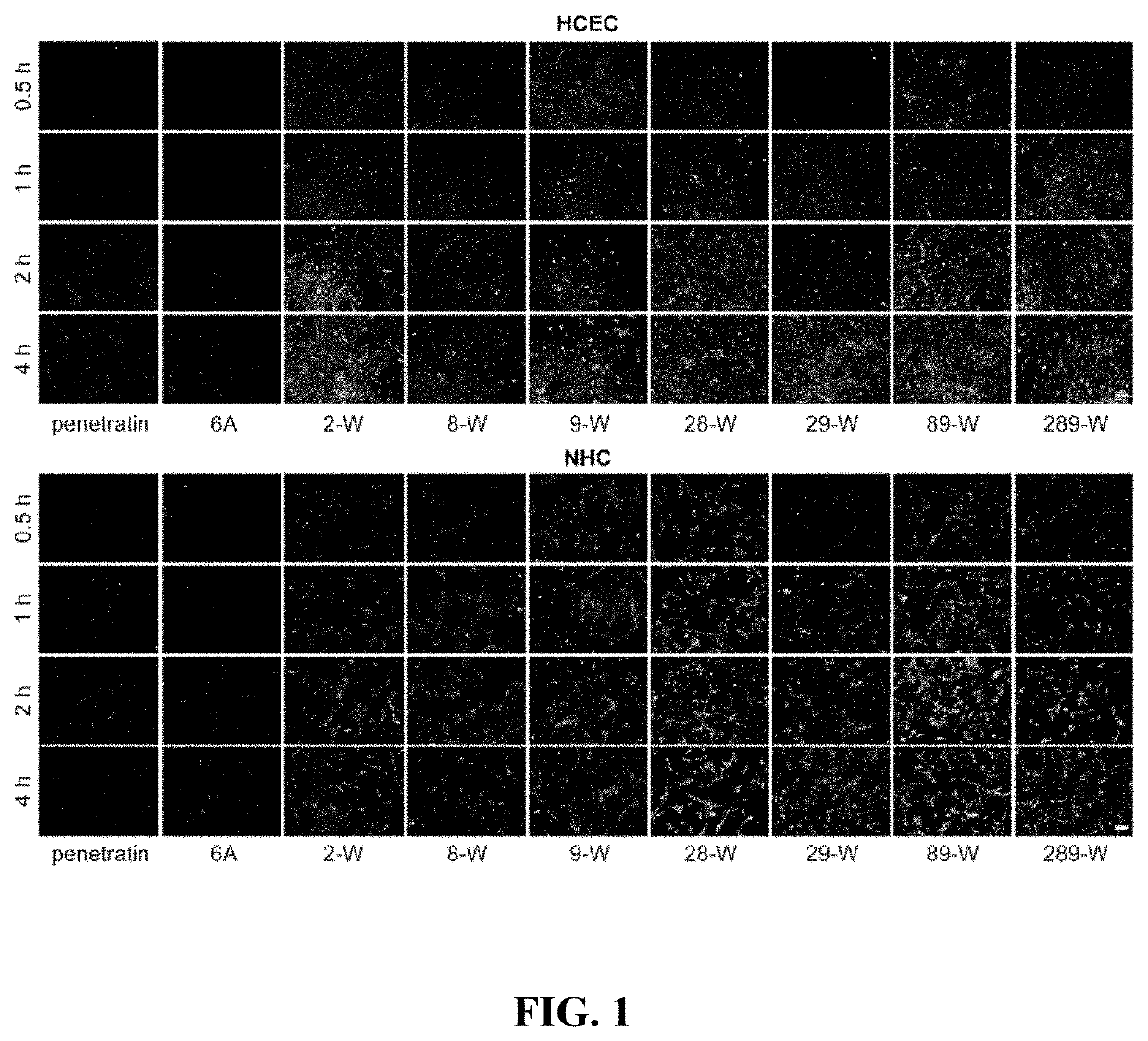
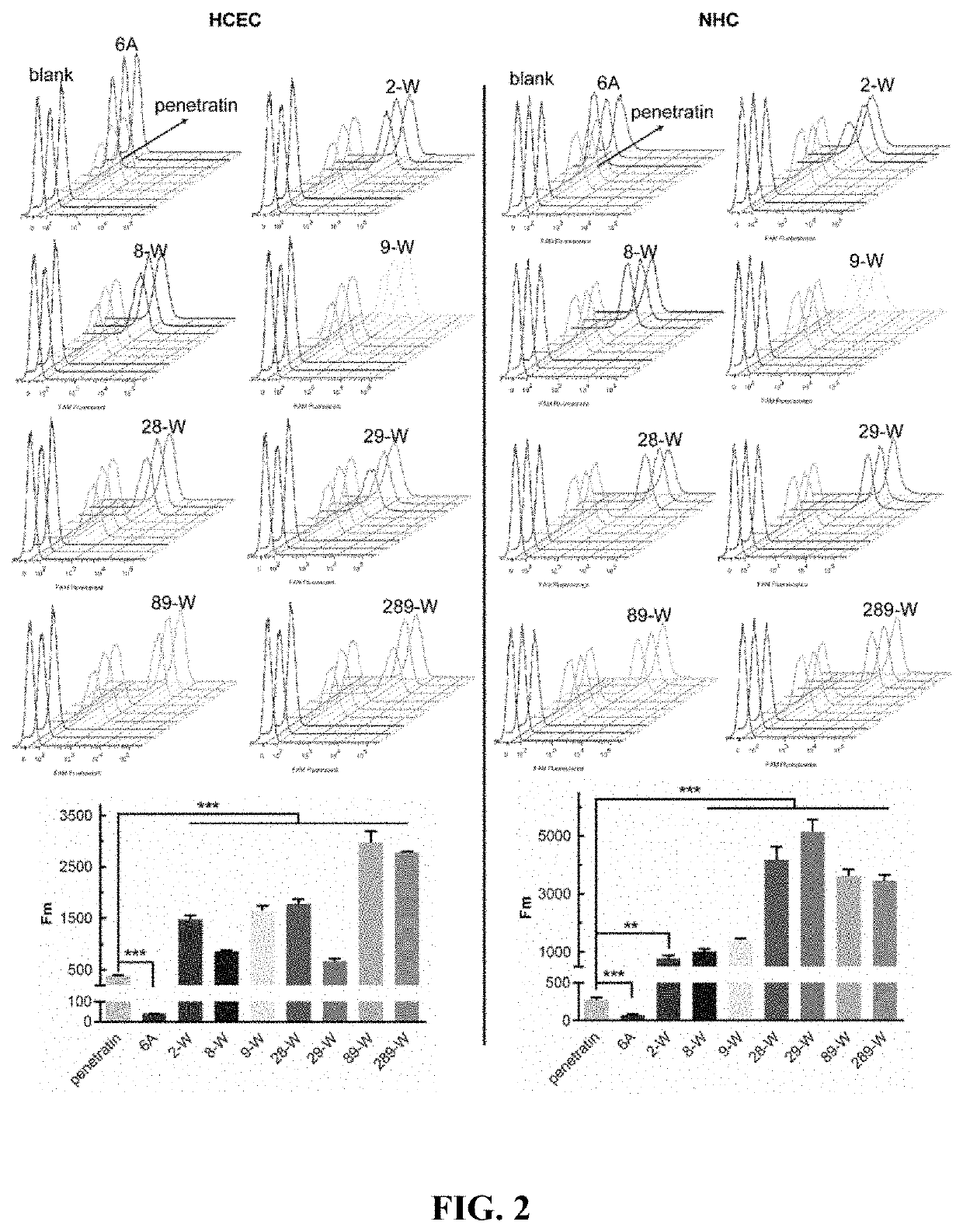
The present disclosure belongs to the field of pharmaceutical preparations and relates to the design of a series of lipophilic derivatives by using wild-type penetrating peptide penetratin. These penetratin derivatives have a strong ability to penetrate the ocular tissues and do not cause ocular tissue toxicity. As ocular absorption enhancers, non-invasive routes could be used to achieve intraocular drug delivery and increase the ocular bioavailability of drugs. These penetratin derivatives and the ophthalmic drug delivery system constructed by them are used for eye drop administration, which could replace the intraocular injection with poor patients compliance, which greatly enhances the convenience and safety of the treatment of intraocular and fundus diseases.
Owner:FUDAN UNIV
Polypeptide eye absorption enhancer and use thereof
ActiveUS20200237925A1Improves ocular bioavailabilityLow complianceAntibacterial agentsSenses disorderOcular bioavailabilityDisease
The present disclosure belongs to the field of pharmaceutical preparations and relates to the design of a series of lipophilic derivatives by using wild-type penetrating peptide penetratin. These penetratin derivatives have a strong ability to penetrate the ocular tissues and do not cause ocular tissue toxicity. As ocular absorption enhancers, non-invasive routes could be used to achieve intraocular drug delivery and increase the ocular bioavailability of drugs. These penetratin derivatives and the ophthalmic drug delivery system constructed by them are used for eye drop administration, which could replace the intraocular injection with poor patients compliance, which greatly enhances the convenience and safety of the treatment of intraocular and fundus diseases.
Owner:FUDAN UNIV
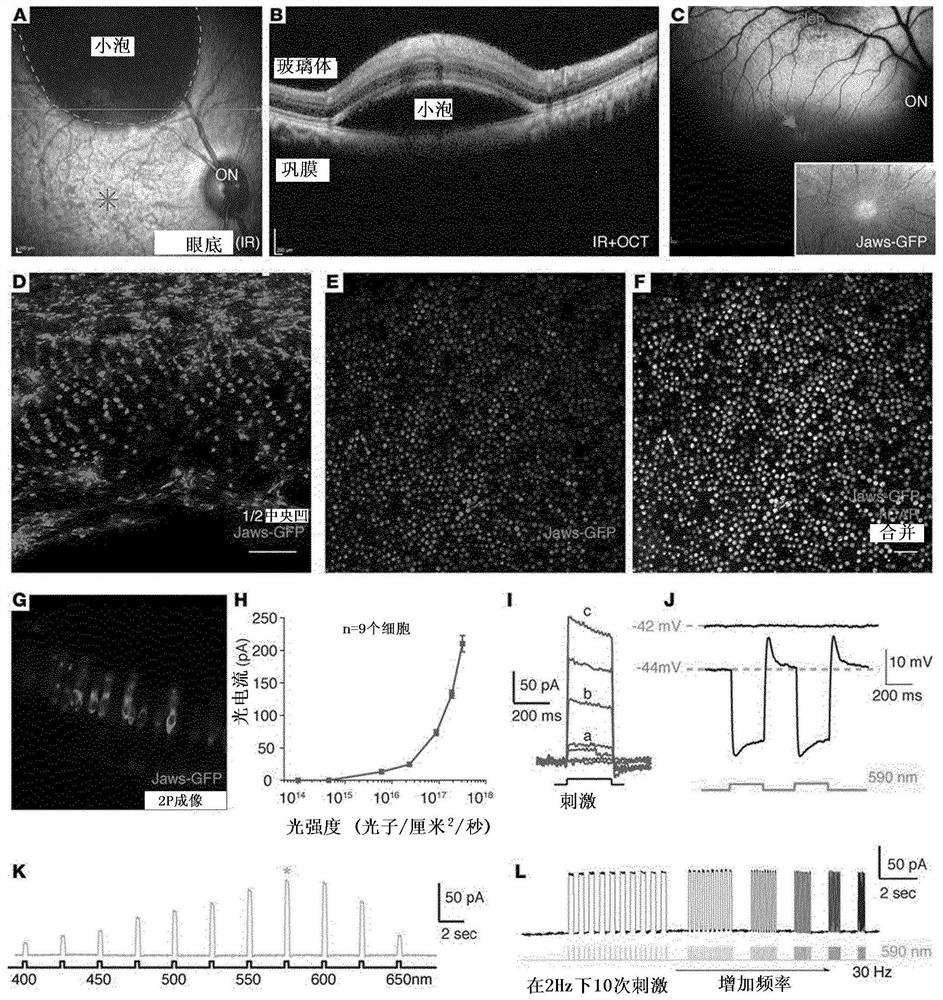
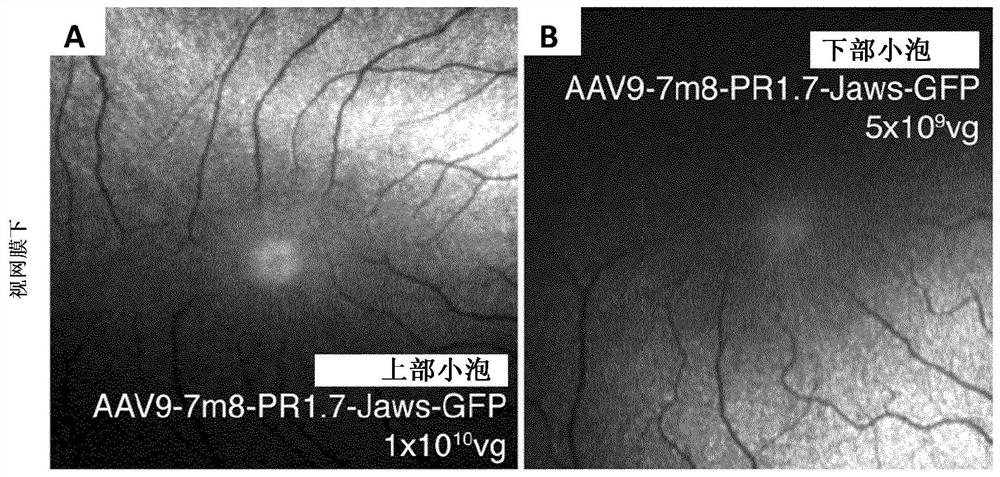
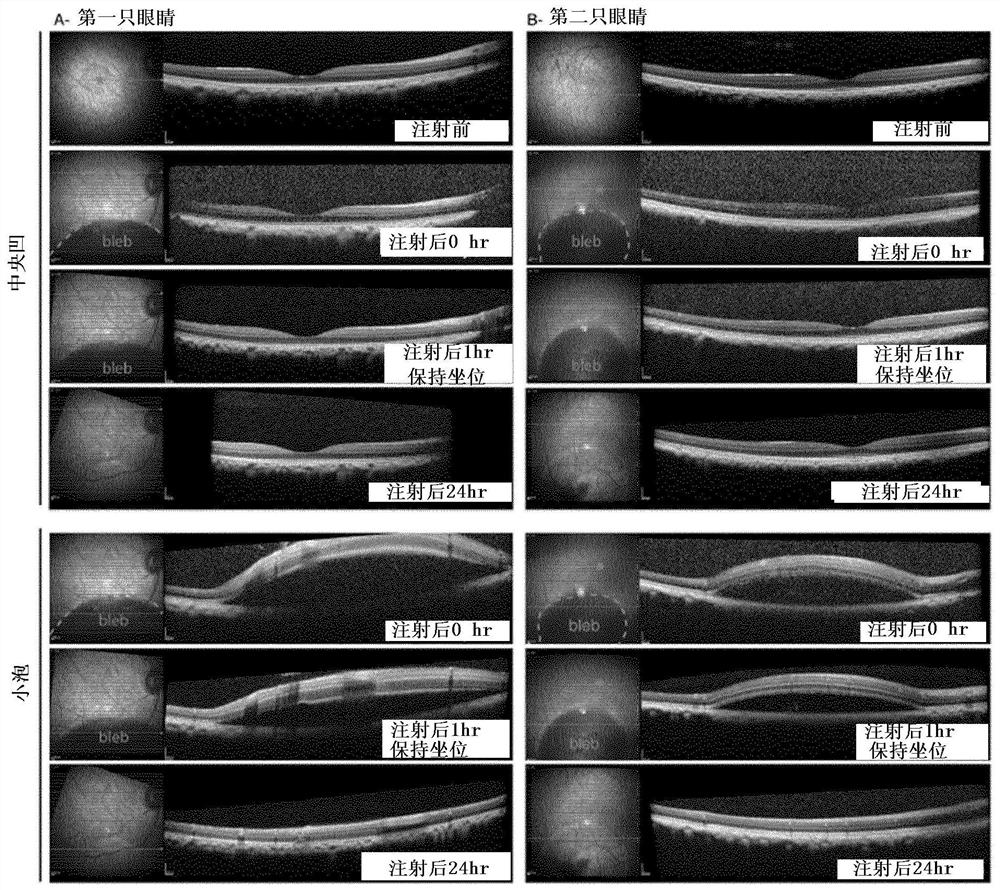
Methods of expressing a polynucleotide of interest in the cone photoreceptors of a subject comprising the subretinal delivery of a therapeutically effective amount of a recombinant aav9-derived vector
Intraocular injection of adeno-associated viral (AAV) vectors has been an evident route for delivering gene drugs into the retina. Currently, the vectors need to be injected into the subretinal spacein order to provide gene delivery to cones. In this approach, gene delivery is limited to cells that contact the local "bleb" of injected fluid. Furthermore, retinal detachment that occurs during subretinal injections is a concern in eyes with retinal degeneration. Here, the inventors establish several new vector-promoter combinations to overcome the limitations associated with AAV-mediated cone transduction in the fovea with supporting studies in mouse models, human induced pluripotent stem cell-derived organoids, post-mortem human retinal explants and living macaques. They show that an AAV9variant provides efficient foveal cone transduction when injected into the subretinal space several millimeters away from the fovea, without detaching this delicate region. The delivery modality relies on a cone-specific promoter and result in high-level transgene expression compatible with optogenetic vision restoration. Accordingly, the present invention relates to method of expressing a polynucleotide of interest in the cone photoreceptors of a subject comprising subretinal delivery of a therapeutically effective amount of a recombinant AAV9-derived vector comprising a VP1 capsid protein asset forth in SEQ ID NO: 11 and the polynucleotide of interest under the control of the pR1.7 promoter as set forth in SEQ ID NO: 12.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS +1
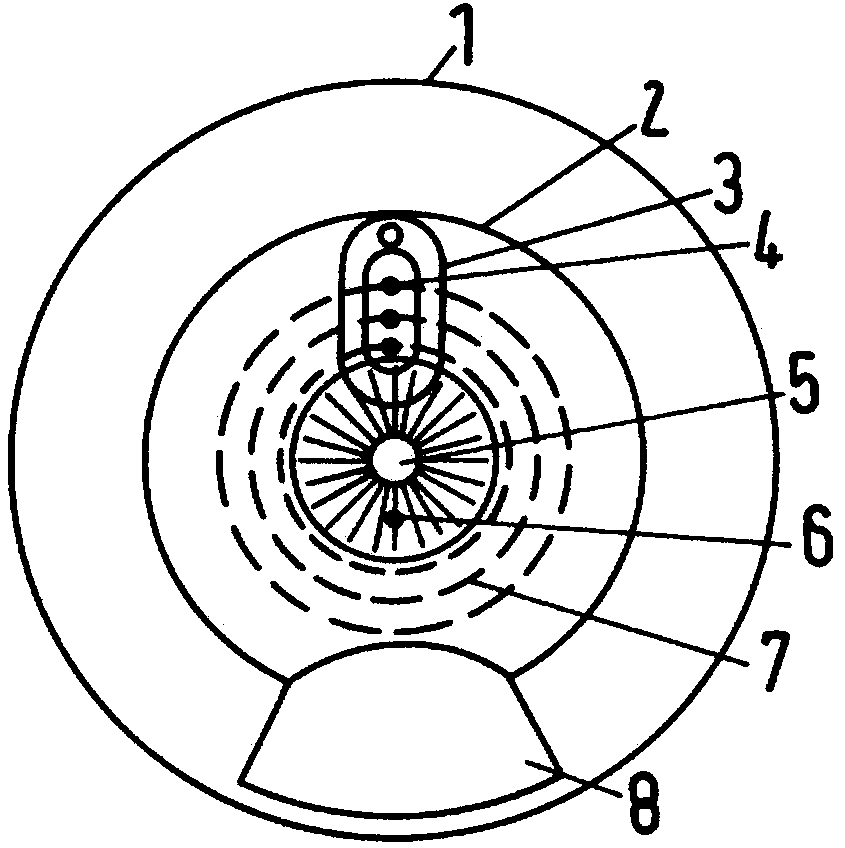
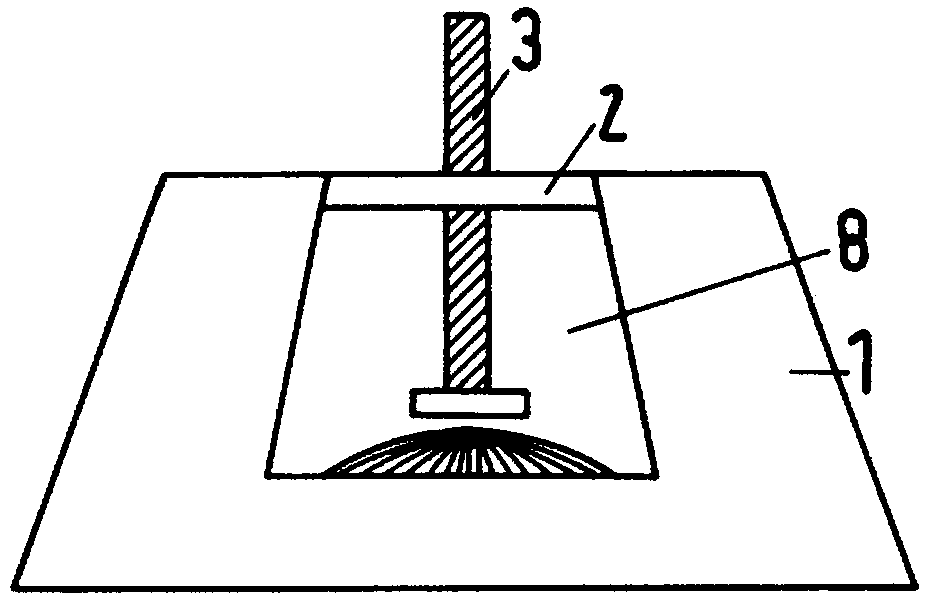
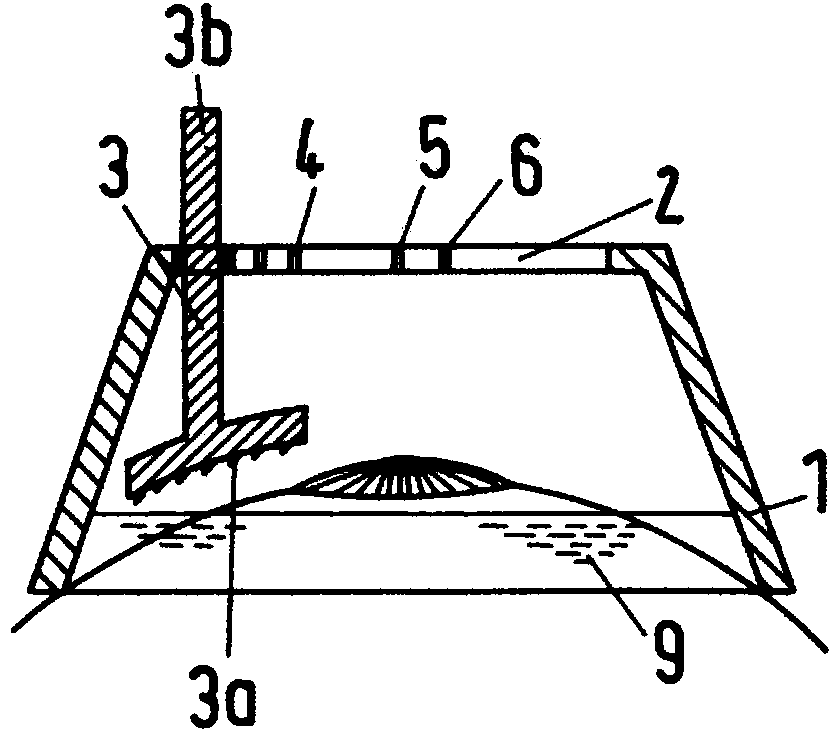
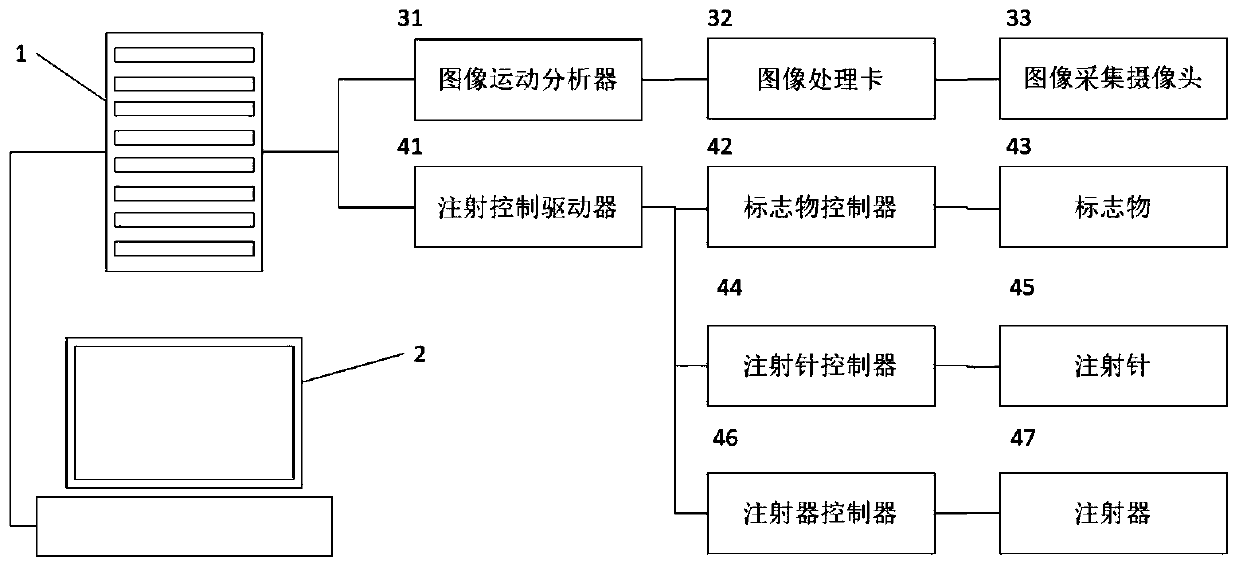
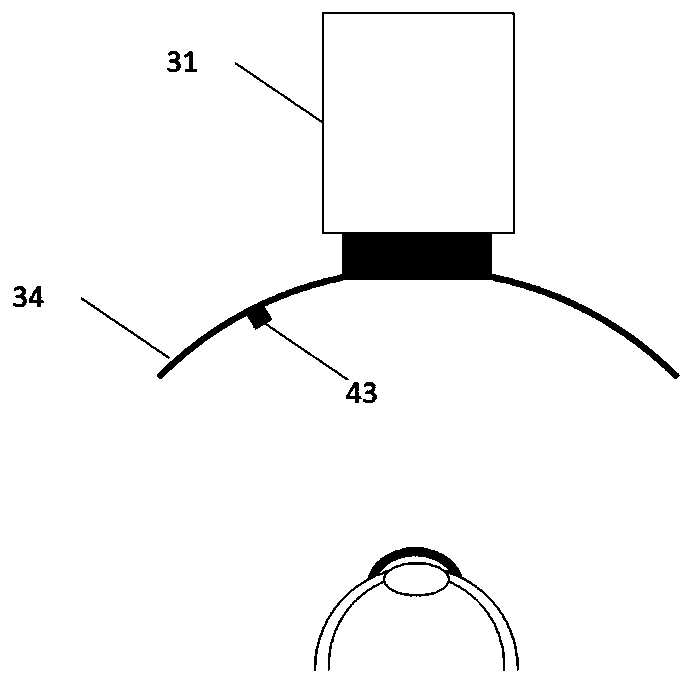
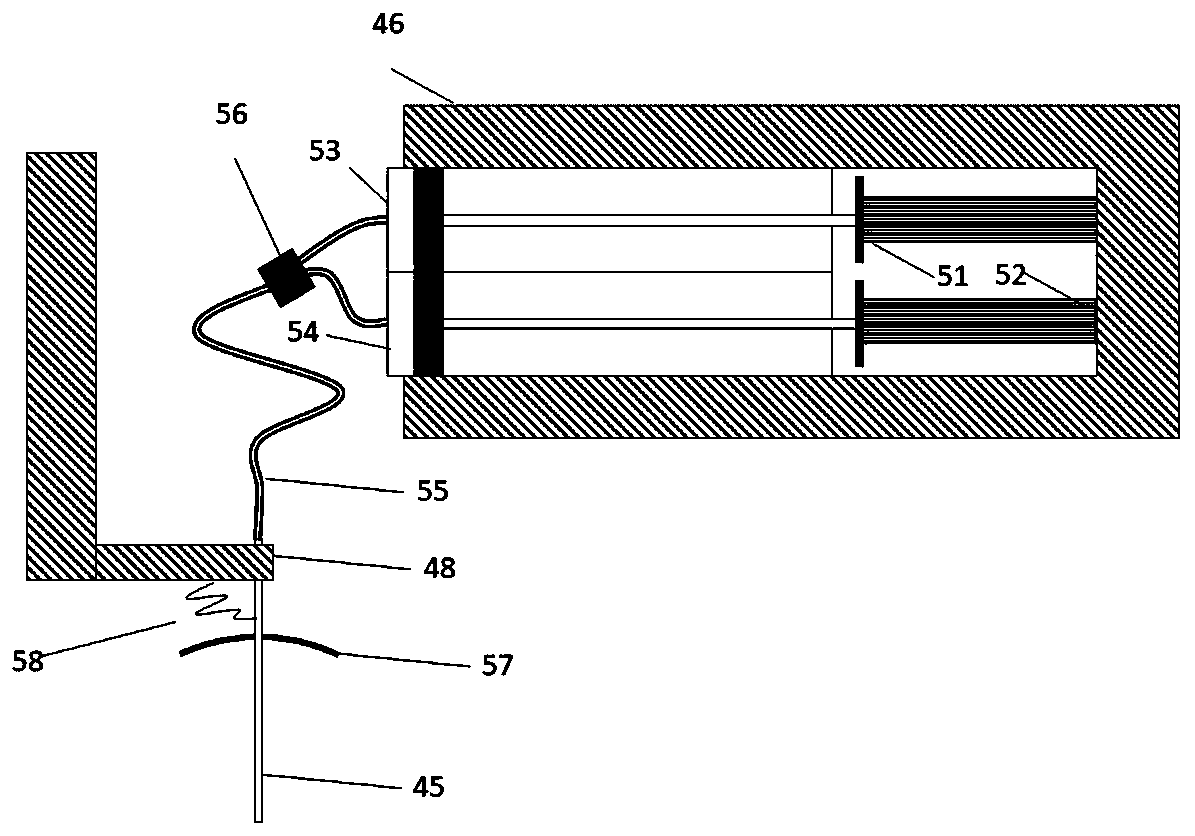
Intelligent intraocular injection device
The invention relates to an intelligent intraocular injection device. The intelligent intraocular injection device is characterized by comprising a control host, a computer, an injection control unitand an image analysis unit; a mark control function is used for controlling the static and moving states of a marker; an injection needle control function is used for inserting an injection needle into an eyeball when the marker is in the static state, and liquid suction and injection operation is conducted. According to the automatically controlled intraocular injection device, the error of manual operation is avoided, the injection operation can be accurately performed in injection coordinates input by the computer, and the amount of injection can be automatically controlled; the injection needle is connected with a needle tube by using a hose, and thus the movement of the eyeball cannot form self-injury to the certain degree; the marker is used for controlling the eyeball position, themarker is watched when using, and thus the eyeball position is more stable; and the injection needle is pulled out by using a self-contained thread after injection to avoid the eye injury.
Owner:曹地
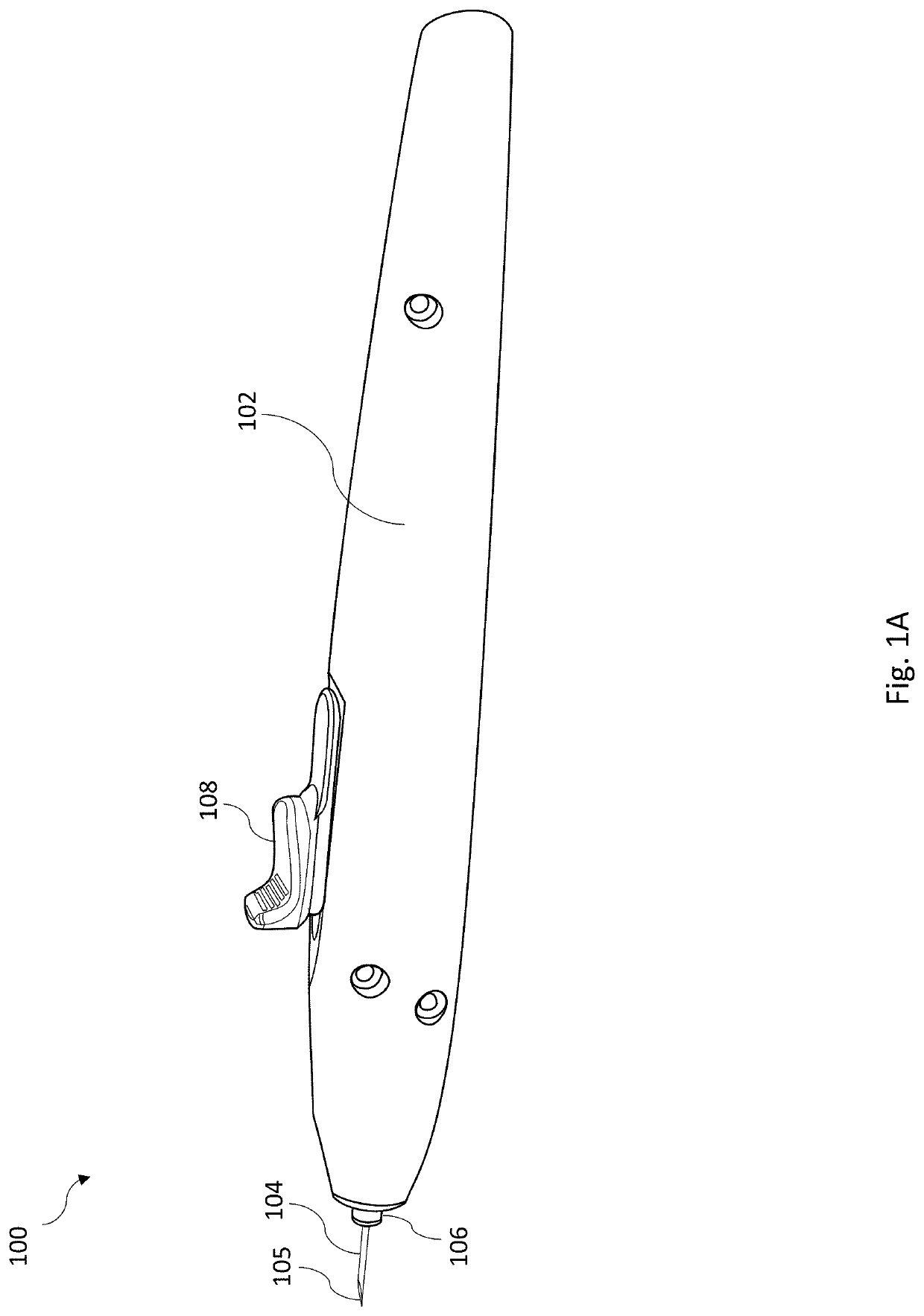
Intraocular injector
Provided herein are assemblies for and methods of intraocular injection. An injector assembly includes a housing defining an internal chamber and having a slot on an external surface thereof; a cannula needle having a lumen and a cutout on an outer surface; a flexible arm having a proximal end affixed to the outer surface of the cannula needle and a distal end having a hook such that the hook is disposed within the cutout; a pushrod slidably disposed within the lumen; and a latch slidably disposed in the slot of the housing, the latch coupled to the pushrod such that translation of the latch causes translation of the pushrod to thereby eject an implant disposed within the lumen between the hook and pushrod.
Owner:INFLAMMASOME THERAPEUTICS INC
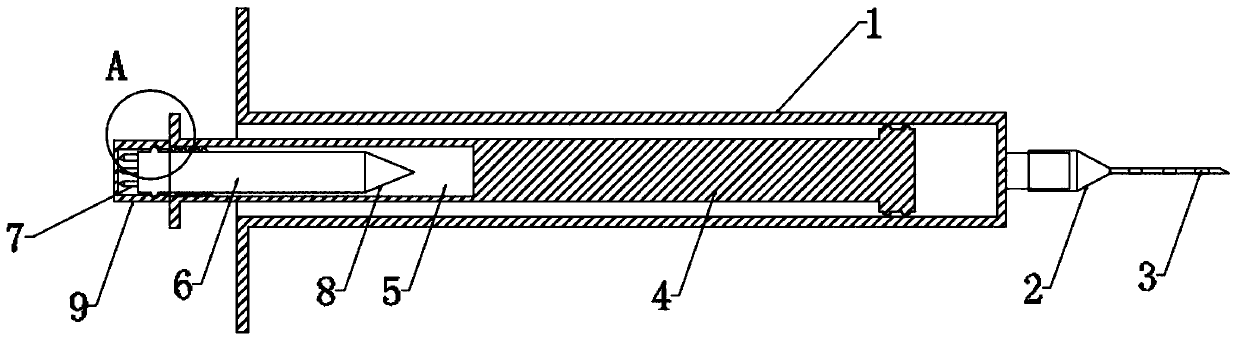
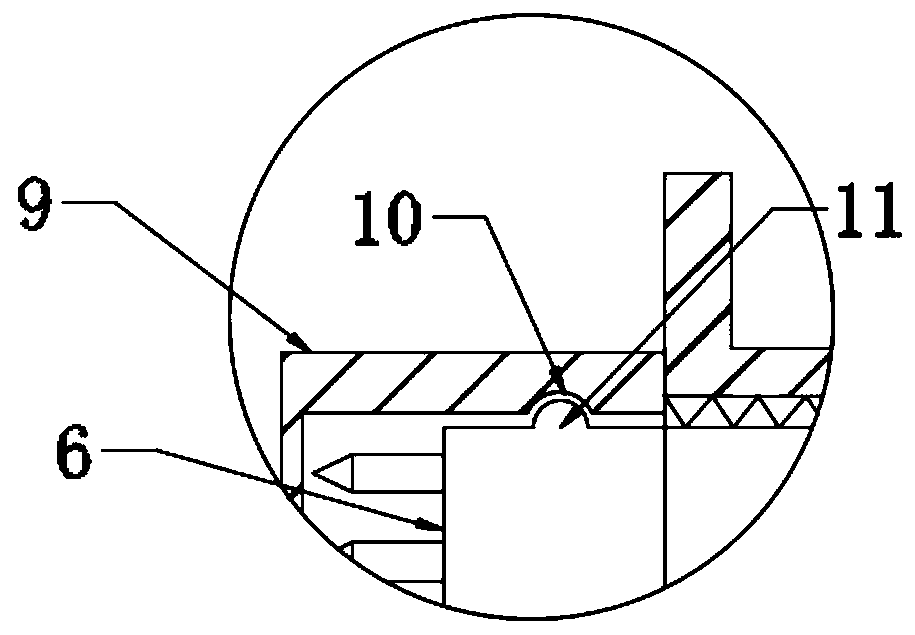
Disposable intraocular injection syringe
The invention belongs to the technical field of medical instruments, and in particular relates to a disposable intraocular injection syringe. The disposable intraocular injection syringe comprises a casing, a syringe needle and a plunger, and a plurality of distance markers can be evenly disposed on the syringe needle for determining the length of the syringe needle insertion. The color of the distance marker is red or orange. The injection syringe solves the problem that the existing intraocular injection does not have any reference for the doctor to refer to, and the doctor is difficult to judge the insertion depth of the syringe needle, which is unfavorable for a surgery. By increasing the depth of the syringe needle insertion by adding a plurality of the distance markers, the depth ofthe syringe needle insertion can be effectively determined, the safety of the intraocular injection is enhanced, and the distance marker is the eye-catching color such as red or orange to help the doctor notice the distance marker more easily.
Owner:苏强 +1
Composition containing ursodeoxycholic acid for prevention or treatment of visual impairment
The present invention relates to a composition containing ursodeoxycholic acid (UDCA) for prevention or treatment of visual impairment. More specifically, the present invention relates to a pharmaceutical composition, which allows oral administration, intraocular injection, or eye drop administration, by aqueous solubilized ursodeoxycholic acid, leading to excellent prevention or treatment effectsof visual impairment-causing diseases, such as macular degeneration, glaucoma, and diabetic retinopathy.
Owner:LIUS BIOPHARMACEUTICALS
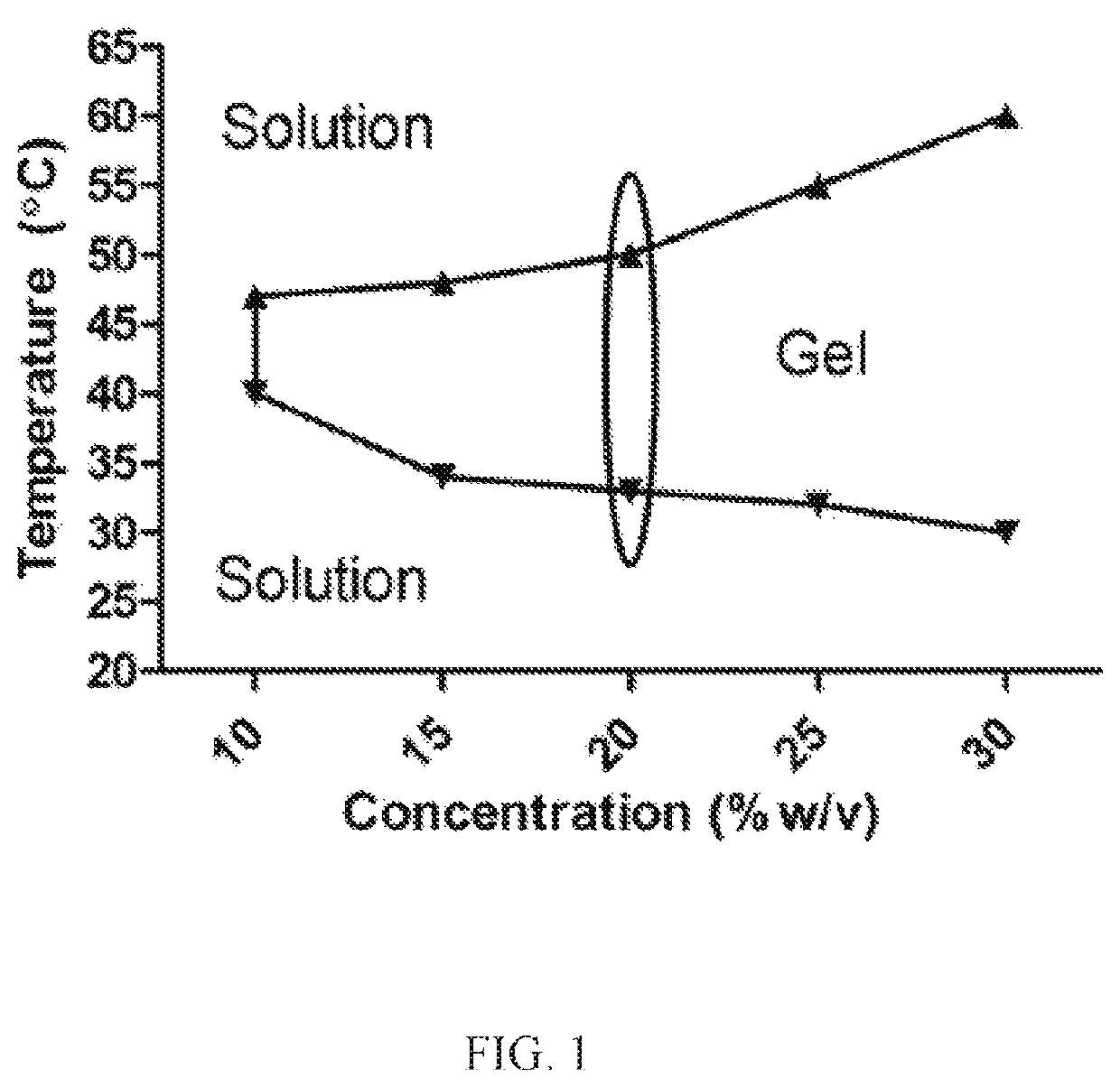
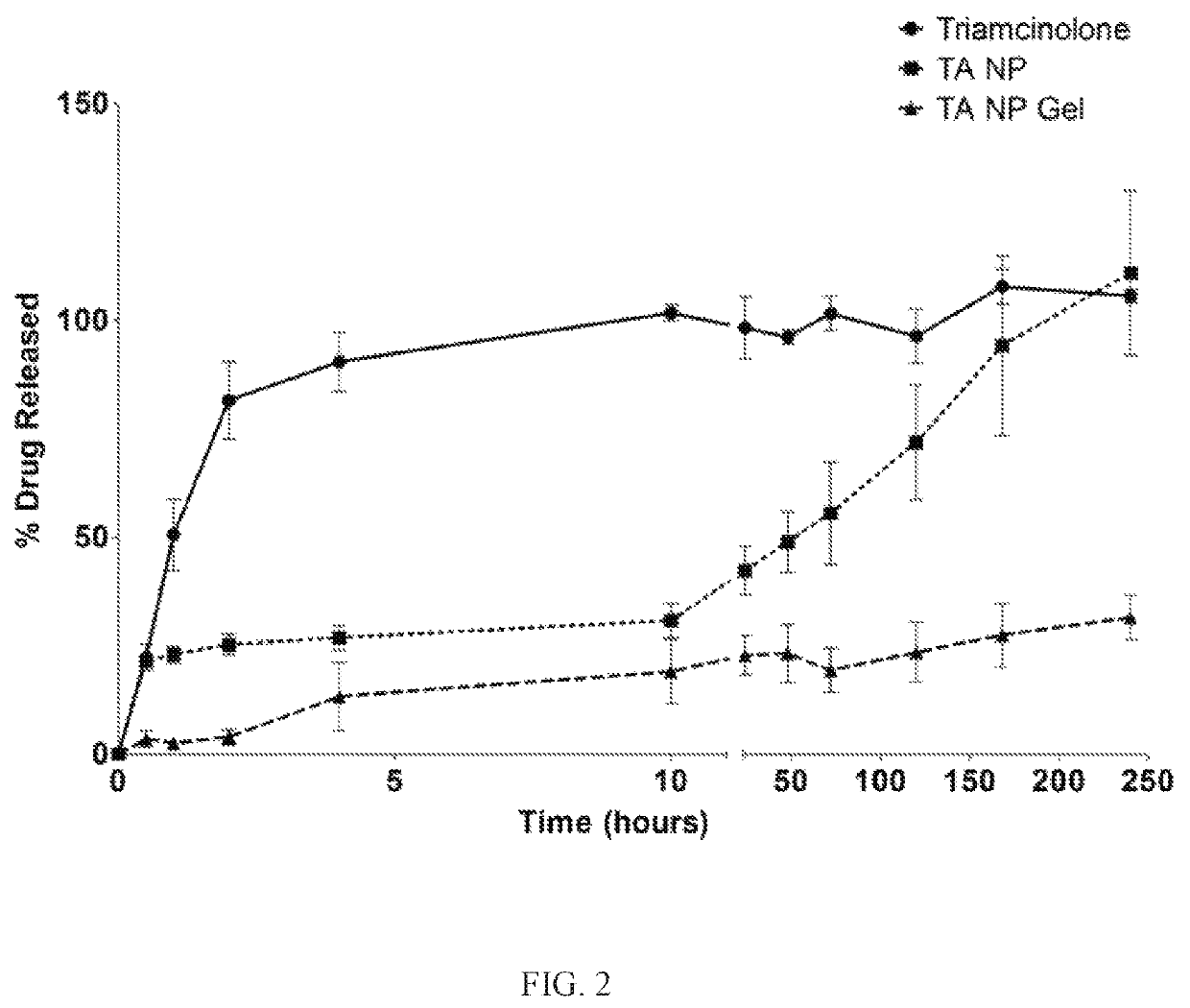
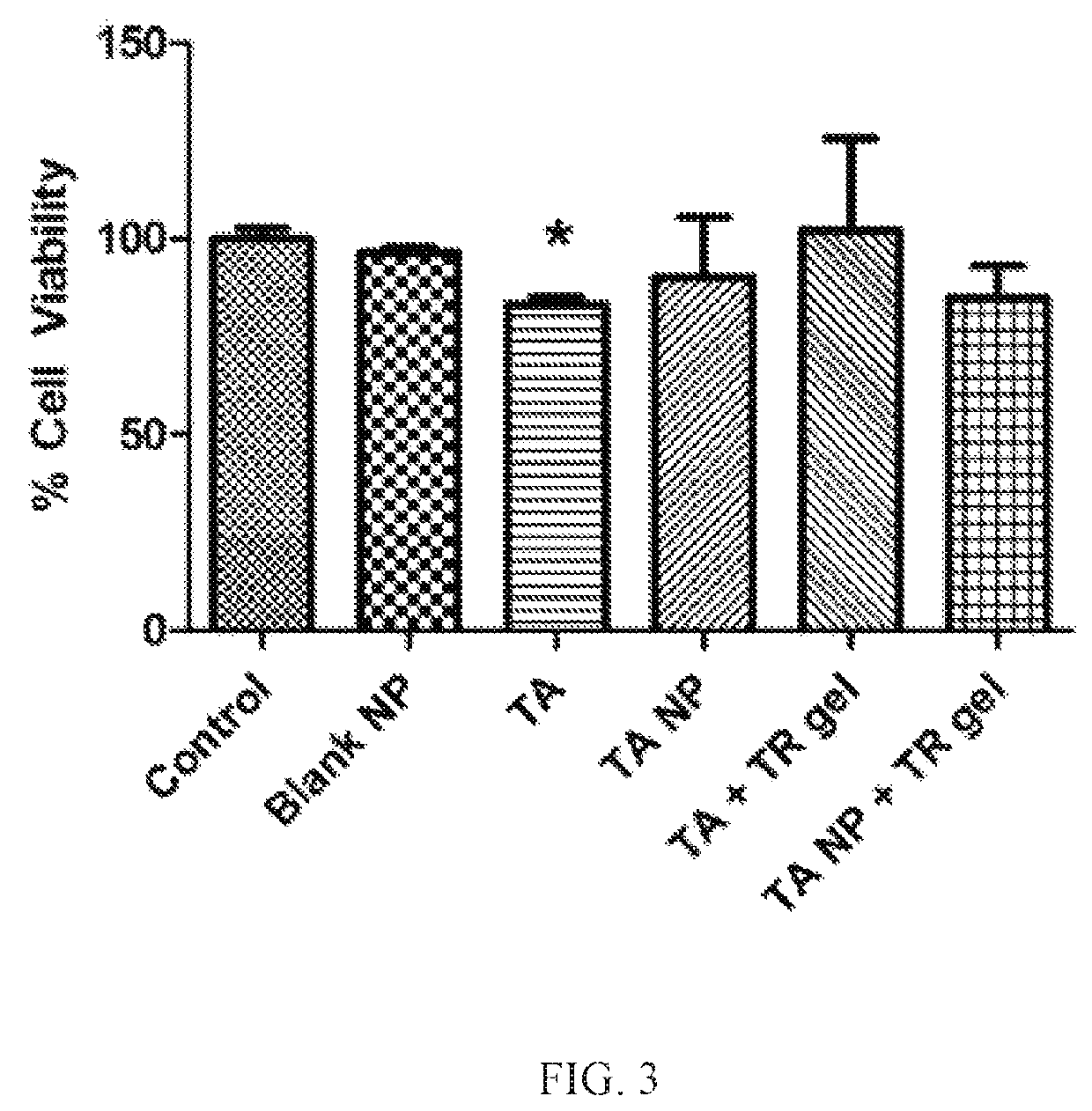
Nanoparticles in thermoreversible gels for enhanced therapeutics
ActiveUS10729663B1Reduce frequencyReduced VEGF expressionOrganic active ingredientsSenses disorderMacula lutea degenerationEye posterior segment
The present invention provides a sustained drug delivery system for the treatment of age-related macular degeneration (AMD), comprising corticosteroid encapsulated nanoparticles incorporated into a thermoreversible hydrogel. The corticosteroid may be triamcinolone acetate (TA), dexamethasone, or loteprednol etabonate (LE). The proposed drug delivery system is nontoxic to ARPE-19 (retinal pigment epithelium) cells and significantly reduces VEGF (vascular endothelial growth factor) expression as compared to solutions of the coticosteroids. The present invention provides sustained delivery of the corticosteroid to the posterior segment of the eye, reducing the frequency of intraocular injections necessary to maintain therapeutic concentrations.
Owner:UNIV OF SOUTH FLORIDA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com