Anti-virus sustained-release drug capable of being subjected to intraocular injection, preparation method and applications thereof
A technology of reaction, foscarnet, applied in the field of antiviral sustained-release drugs and their preparation, can solve the problems of high dosage of drugs, inability to apply the degradable microsphere sustained-release system, etc., and achieve the effect of good health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
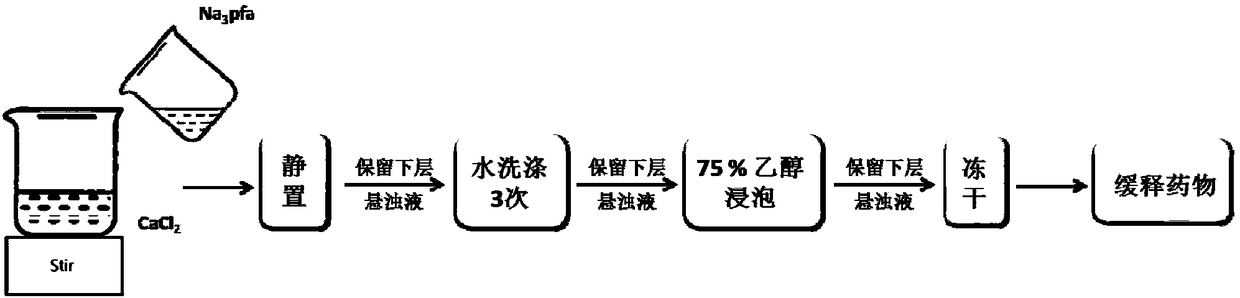
[0034] Embodiment 1, the preparation of foscarnet insoluble salt micro-crystal
[0035] according to figure 1 The preparation flow chart shown is to prepare micro-crystals of foscarnet insoluble salt.
[0036] Prepare 2mg / mL sodium foscarnet aqueous solution and 2mg / mL calcium chloride solution, pour the two solutions with a volume ratio of 1:1 and mix them under magnetic stirring, and continue stirring for 1 hour after mixing; stop stirring, and let it stand for 10 minutes. Slowly pour off the supernatant; wash the sample 3 times with pure water; freeze-dry the suspension to obtain product a.
[0037] According to the above method, by adjusting the concentration of foscarnet sodium solution and calcium chloride solution, or by adjusting the mixing volume ratio of foscarnet sodium solution and calcium chloride solution, foscarnet insoluble salt micron crystal slow-release medicines of different sizes can be prepared.
[0038] According to the above preparation method, a 6 mg...
Embodiment 2
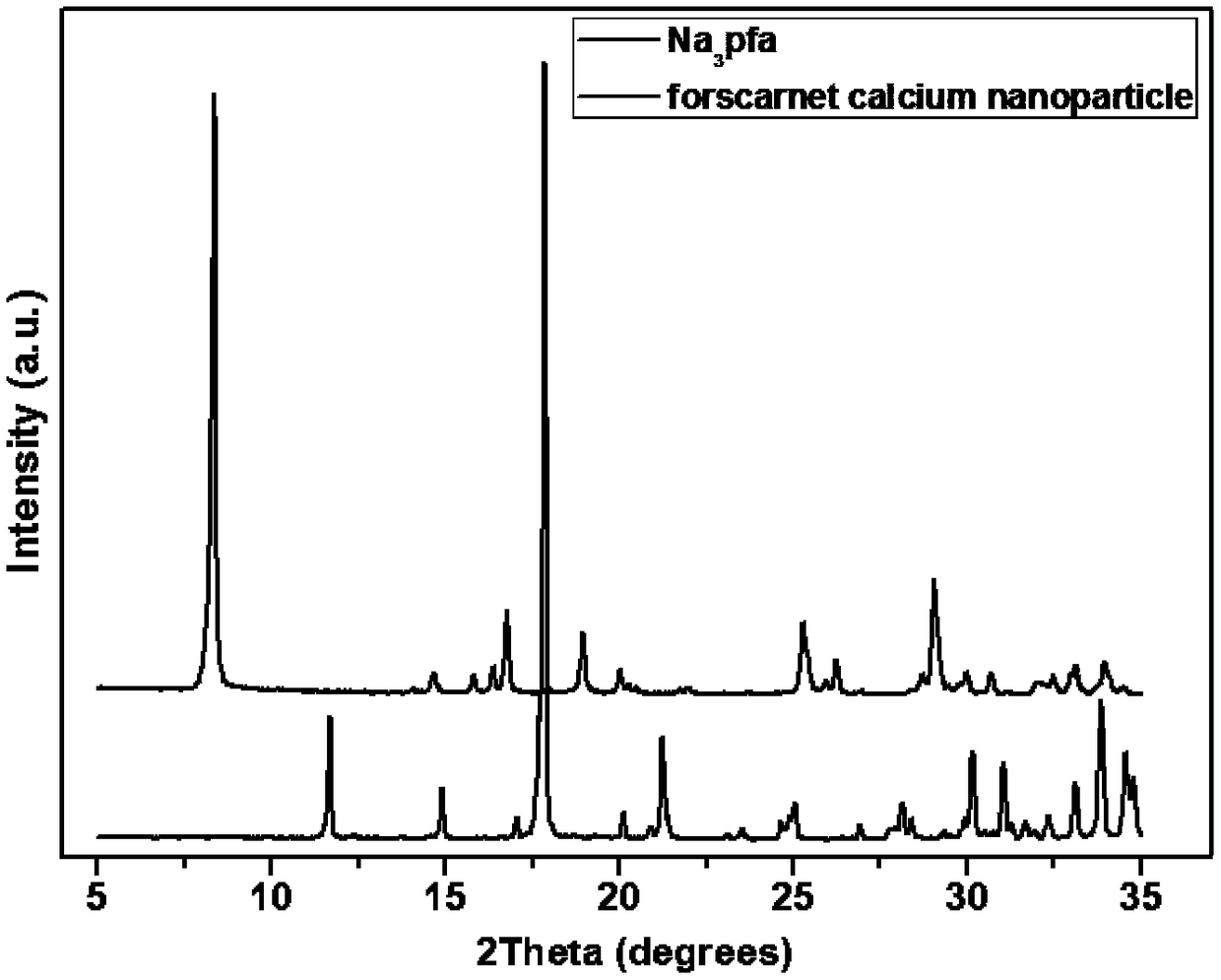
[0040] Embodiment 2, in vitro performance characterization of foscarnet insoluble salt micro-crystals
[0041] Scanning electron microscopy characterization: the freeze-dried powder samples a, b, and c of the micro-crystals of foscarnet refractory salt prepared in Example 1 were evenly sprinkled on the sample stage with conductive glue, and the excess samples on the surface were blown off. Scanning electron microscope observation. See the experimental results figure 2 .
[0042] figure 2 (a), (b) and (c) are the scanning electron microscope pictures of the micro-crystals a, b, and c of the refractory salt of foscarnet respectively, and the average sizes are estimated to be 10 μm, 60 μm, and 100 μm in diameter, respectively, and the crystals can be seen Both grow lamellar crystals radially from the center to form spherical crystals of micron size.
[0043] Plasma mass spectrometry characterization: Weigh 50 mg of foscarnet insoluble salt micron crystal freeze-dried powder...
Embodiment 3
[0050] Embodiment 3, animal intraocular sustained release experiment of foscarnet insoluble salt micro-crystal
[0051] In the experiment, 13 New Zealand white rabbits were selected, and both eyes were injected with slow-release drugs.
[0052] The animal experiment steps are as follows:
[0053] 1. The formulation of the injection suspension: 180mg of foscarnet insoluble salt slow-release drug (product a) was dissolved in 1.8mL 1% CMV solution (carboxymethylcellulose), before vortex mixing, slow-release drug and CMV solution All should be irradiated with ultraviolet light for 60 minutes to carry out disinfection and sterilization.
[0054] 2. New Zealand white rabbits were anesthetized by intravenous injection of pentobarbital.
[0055] 3. Inject foscarnet insoluble salt / CMV suspension into the vitreous cavity of both eyes of white rabbits with a 29G insulin needle. After the injection, a white substance was observed in the vitreous cavity, confirming that the injection was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com