Long-acting sustained release preparation for preventing or treating retinal damage, and preparation method thereof
A technology for retinal damage and sustained-release preparations, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of not being able to replace systemic medication, and achieve the effect of alleviating pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
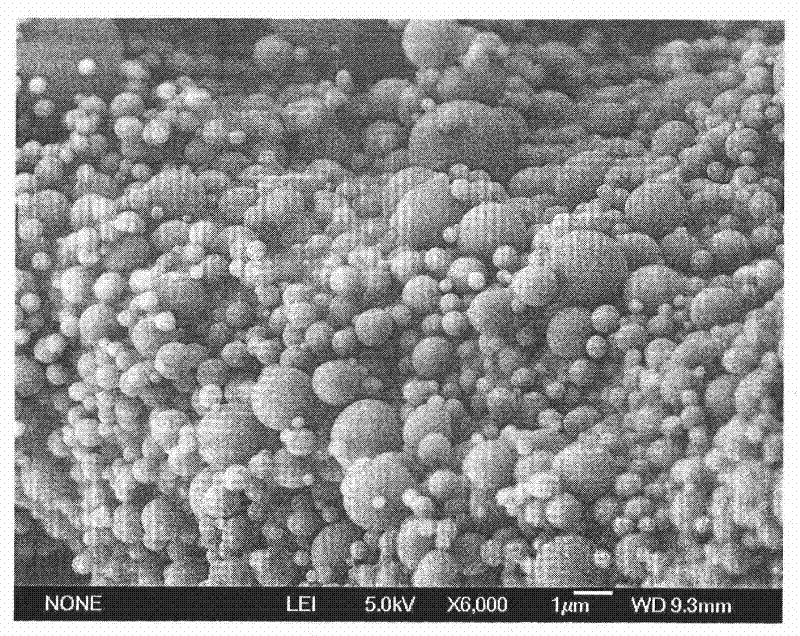
[0052] ①Preparation of erythropoietin-dextran microparticles
[0053] a) Pre-preparing erythropoietin with a weight percentage of 0.5%, 10%, or 20% of the erythropoietin microspheres into a solution with a weight percentage concentration of 0.01%, 10%, or 20%, respectively, Dextran (with a molecular weight of 10,000, 2,500,000, or 5,000,000 Daltons) accounted for 0.5%, 15%, or 30% by weight of the erythropoietin microspheres was formulated to have a concentration of 1%, 15% by weight, respectively. % or 30% solution; polyethylene glycol (molecular weight: 2,000, 150,000, or 300,000) is prepared into a solution with a concentration of 1%, 20%, or 40% by weight respectively.
[0054] b) Mix the above erythropoietin solution according to the volume ratio of erythropoietin solution: dextran solution: PEG solution = 50:1:200, 1:1:8 or 2:3:12 and mix well ;
[0055] c) Then mix the above step b) evenly, pre-freeze in the freezer for 8-32 hours, and then freeze-dry,
[0056] d) Di...
Embodiment 2
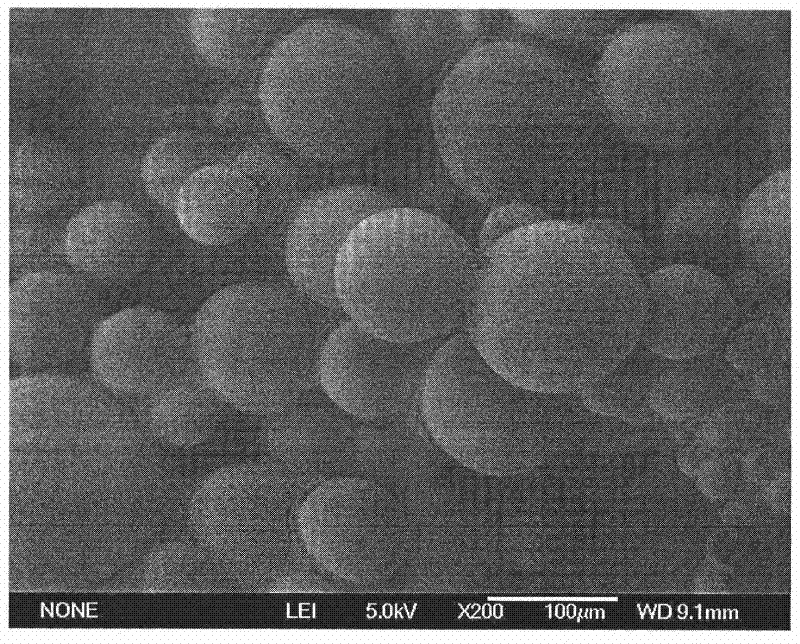
[0064] ①Preparation of erythropoietin-dextran microparticles
[0065] With step 1. of embodiment 1.
[0066] a) Take 1 mg, 2.5 mg or 3 mg of the erythropoietin microparticles prepared in the corresponding proportion of step ① and add them to 330 mg of polylactic acid-glycolic acid (PLGA), polylactic acid (PLA) with a concentration of 30% by weight. , or a dichloromethane solution of polycaprolactone (PCL) (the prepared erythropoietin sustained-release microspheres contain 0.5% erythropoietin and 0.5% dextran), and the weight percent concentration is 15% 50mg of polylactic acid-glycolic acid (PLGA), polylactic acid (PLA), or polycaprolactone (PCL) in dichloromethane solution (the prepared erythropoietin sustained-release microspheres contain 10% erythropoietin and 15% dextran), or 5% of 140mg polylactic-glycolic acid (PLGA), or polycaprolactone (PCL) in dichloromethane solution (the prepared erythropoietin sustained-release microspheres containing 20% erythropoietin and 30% d...
Embodiment 3
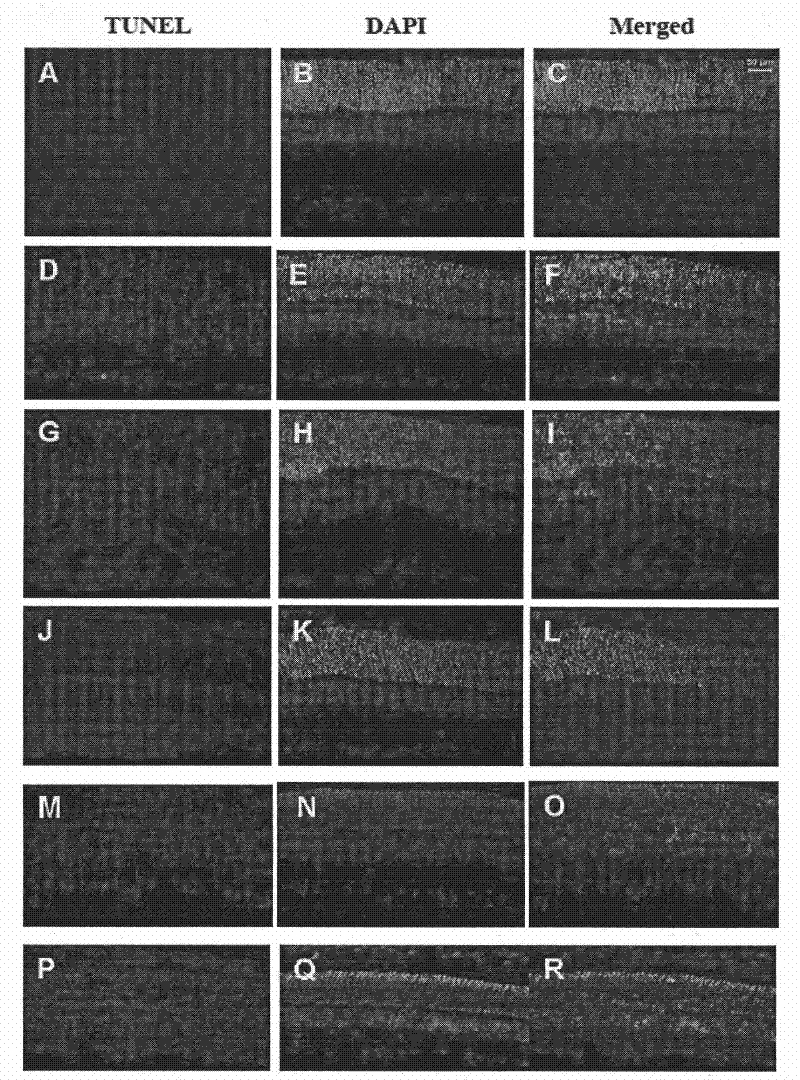
[0072] Apoptosis in situ cell apoptosis detection method (TUNEL detection) to observe the protective effect of the long-acting sustained-release preparation EPO sustained-release microspheres of the present invention on damaged retinal ganglion cells (RGCs)
[0073] ①Experimental animals and grouping: Adult male SD rats (about 200g), after 7 days of adaptive feeding, check both eyes, check that the refractive interstitium is clear, the pupils are equal in size and round, sensitive to light reflex, and the fundus is normal. The left eye was regarded as the optic nerve contusion eye, and the right eye was regarded as the normal control eye. According to the intravitreal injection administration after optic nerve contusion, they were divided into the following five groups: untreated group (no injection into vitreous cavity), EPO group (injection of EPO into vitreous cavity), EPO-PLGA group (injection of EPO-PLGA microspheres into vitreous cavity) , PBS group (intravitreal injecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com