Patents
Literature
45results about How to "Low compliance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Systems and Methods for Providing Percutaneous Electrical Stimulation
ActiveUS20130096641A1Easy to implementReduce riskElectrotherapyArtificial respirationTreatment painPeripheral neuron
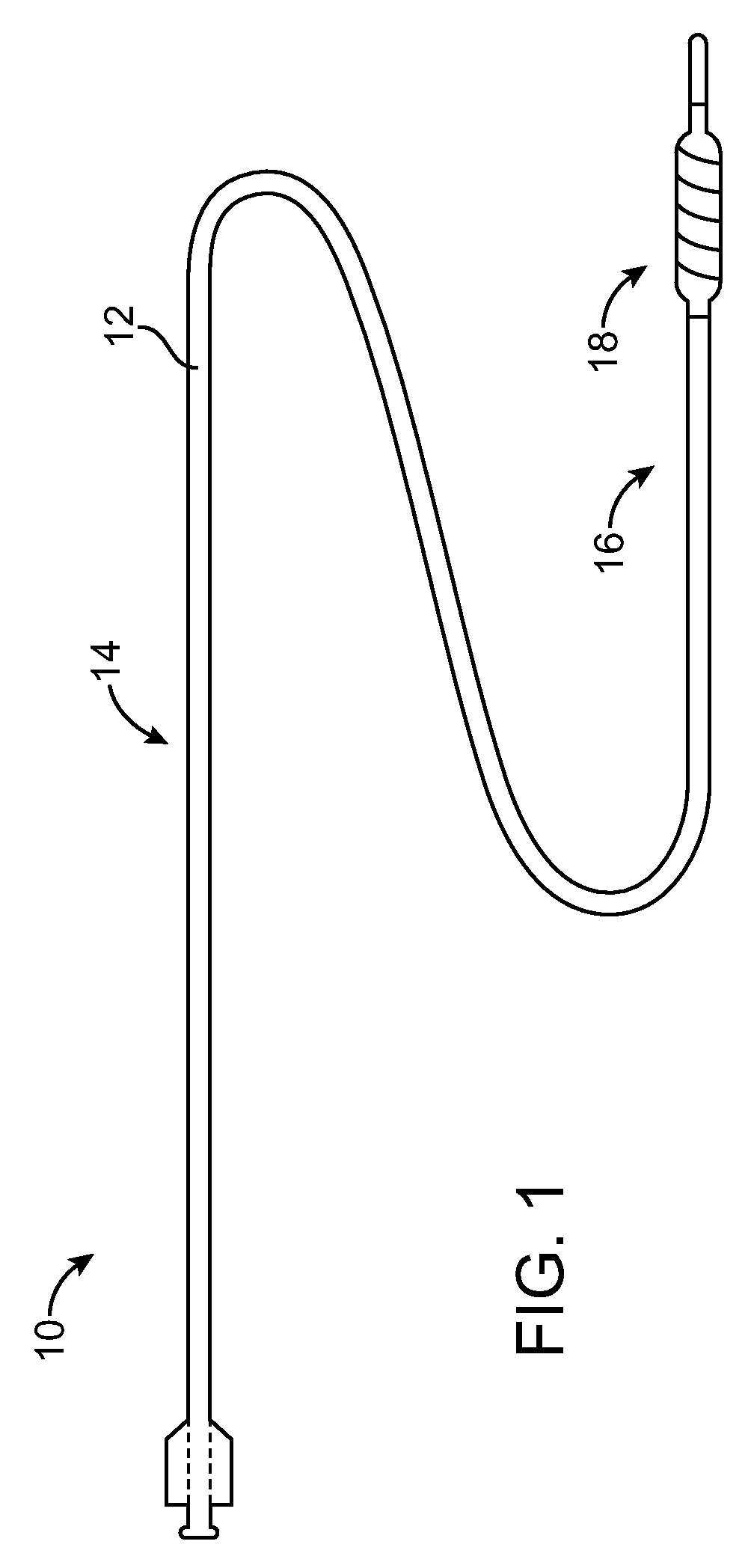
Systems and methods according to the present invention relate to a novel peripheral nerve stimulation system for the treatment of pain, such as pain that exists after amputation.
Owner:SPR THERAPEUTICS
Method for providing high bandwidth force feedback with improved actuator feel
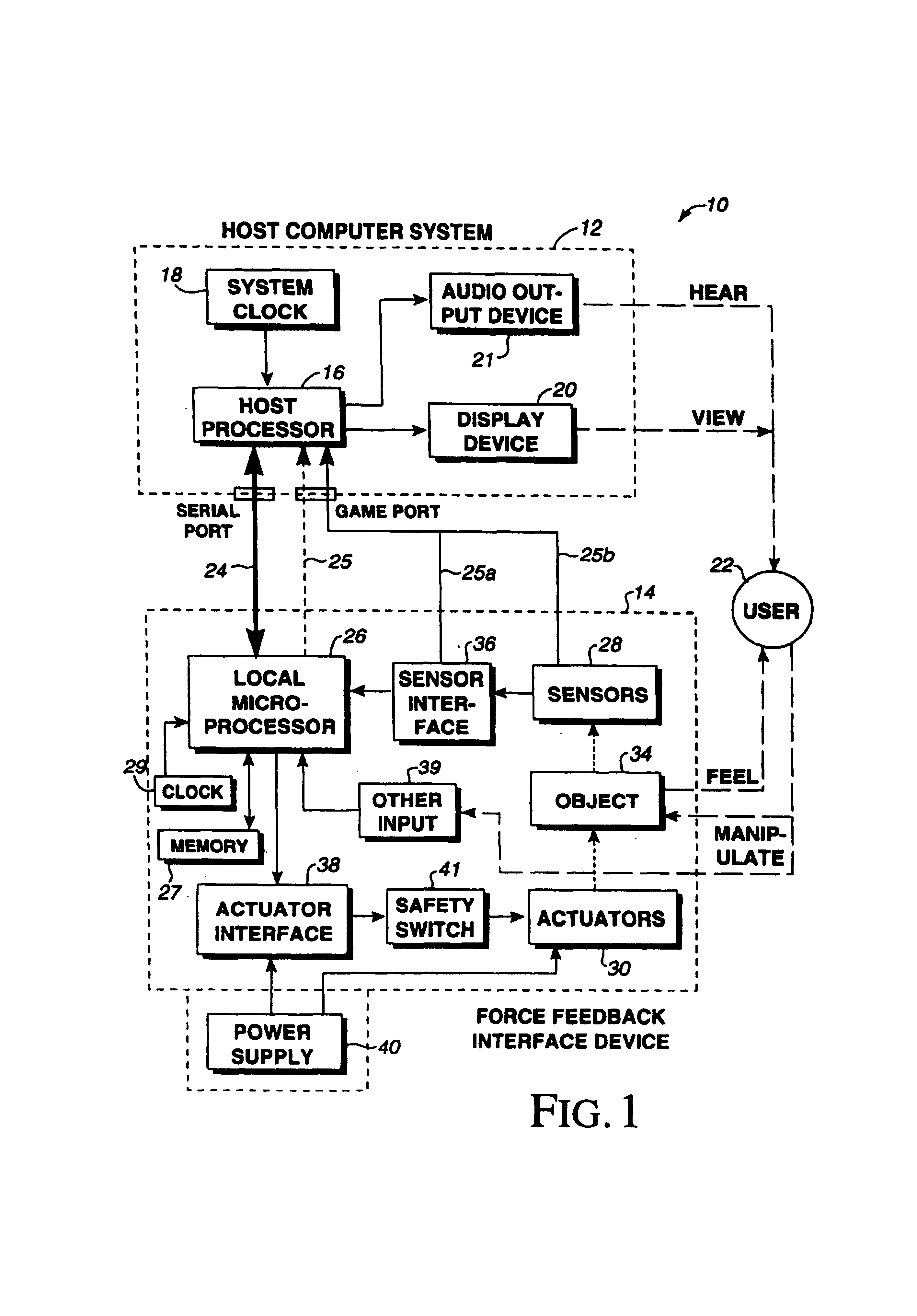
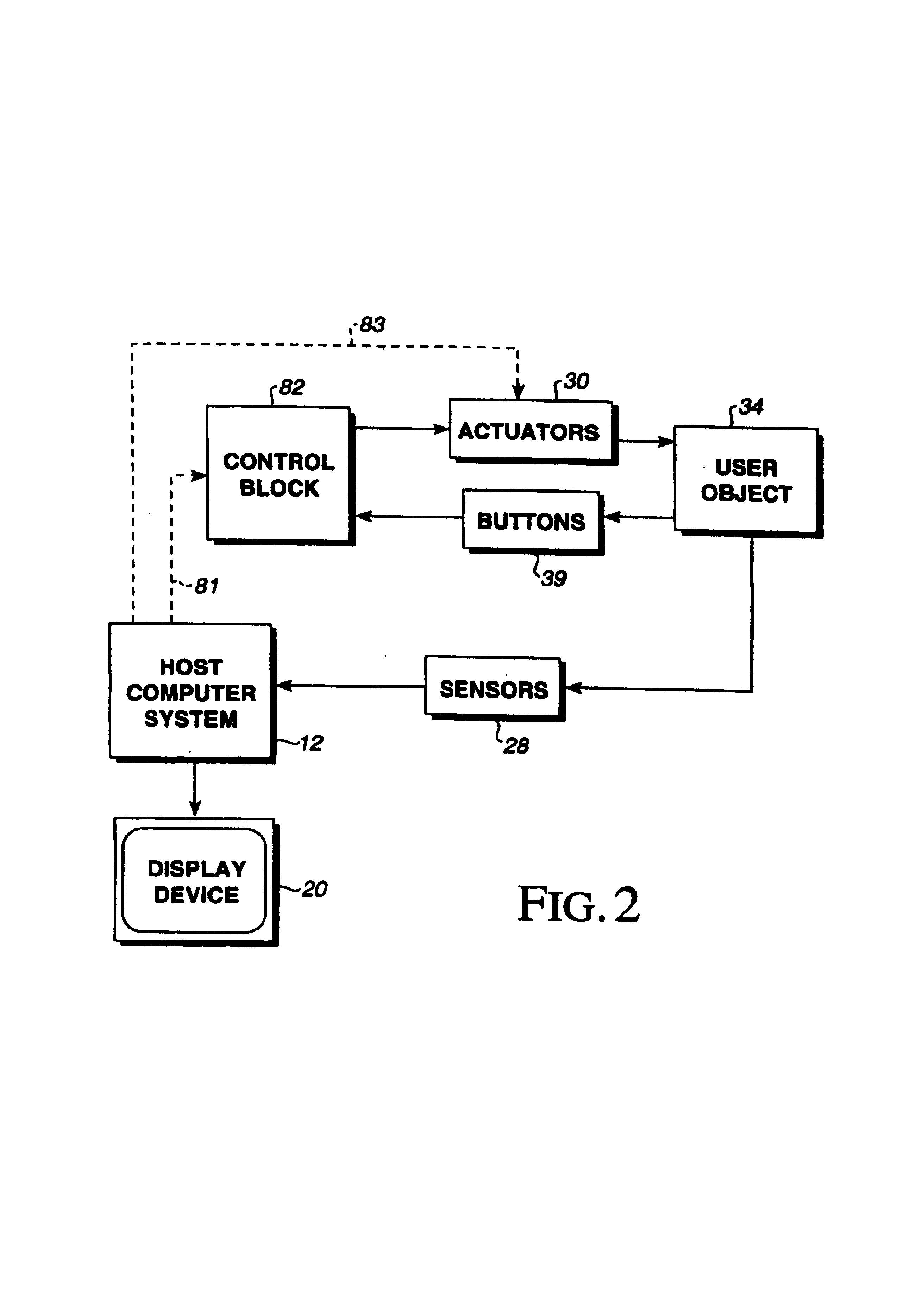
InactiveUS7236157B2Improve realismEliminate the effects ofInput/output for user-computer interactionManual control with multiple controlled membersHigh bandwidthJoystick
A method and apparatus for providing low-cost, realistic force feedback including an improved actuator. The invention provides force sensations to a user and includes an interface device coupled to a host computer and allowing a user to interact with a host application program. A user object, such as a joystick, is moveable by a user in at least one rotary degree of freedom. A sensor reports a locative signal to the host computer to indicate a position of the user object. An actuator outputs forces on the user object in response to signals from the host computer and program. The actuator includes a housing, a set of grounded magnets provided on opposing surfaces of the housing and creating a magnetic field, and a rotor coupled to the user object positioned between the magnets. The rotor rotates about an axis of rotation and includes a shaft and teeth spaced around the shaft. An electric current flows through one or more coils on the teeth to cause the rotor to rotate. The teeth and the magnets are provided in a skewed, helical arrangement relative to each other so that, as the rotor rotates, a first tooth gradually exits the magnetic field as the next consecutive tooth gradually enters the magnetic field, thereby significantly reducing a cogging effect of the rotor when the user object is moved by the user and increasing the fidelity of forces experienced by the user.
Owner:IMMERSION CORPORATION
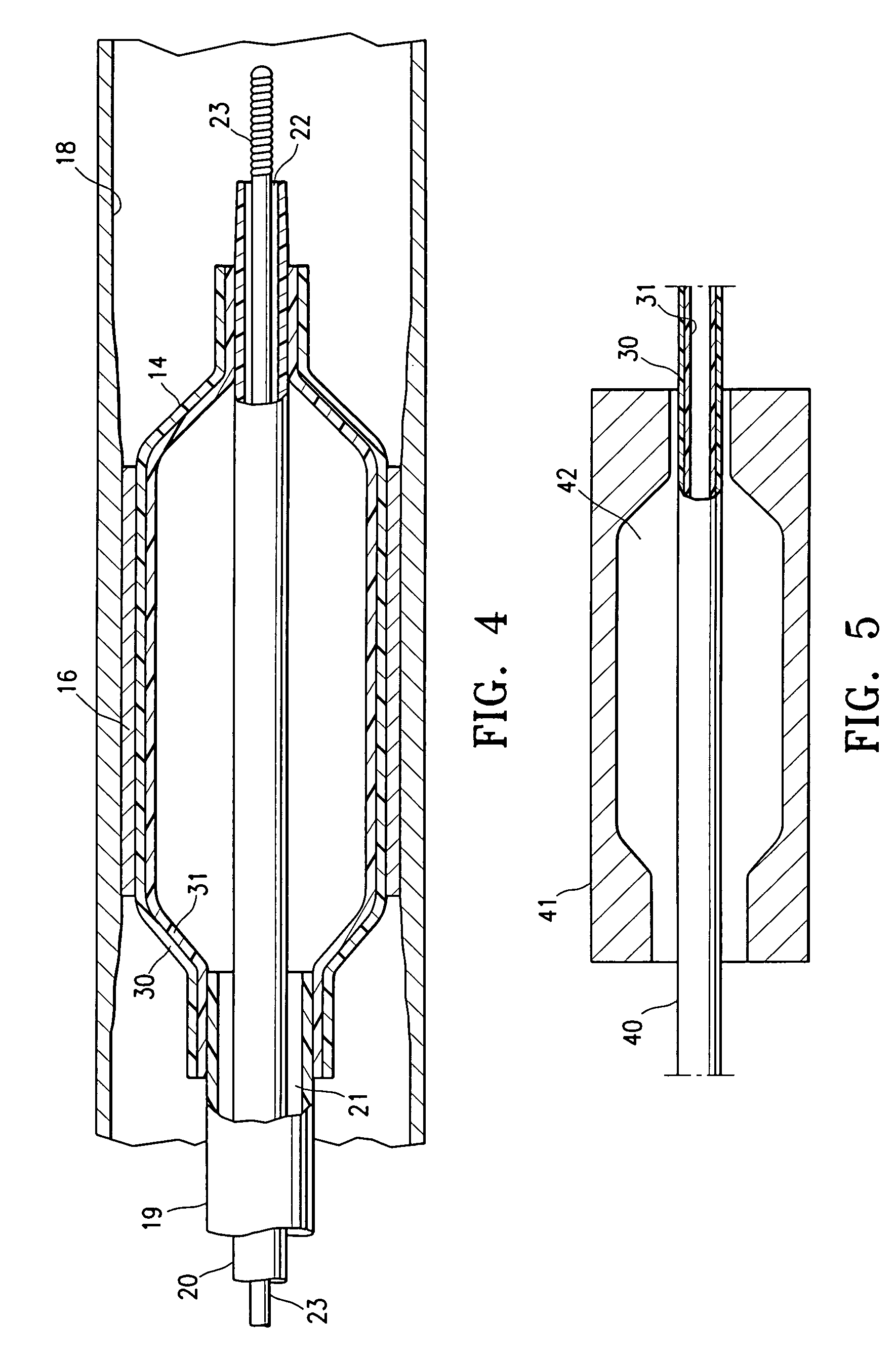
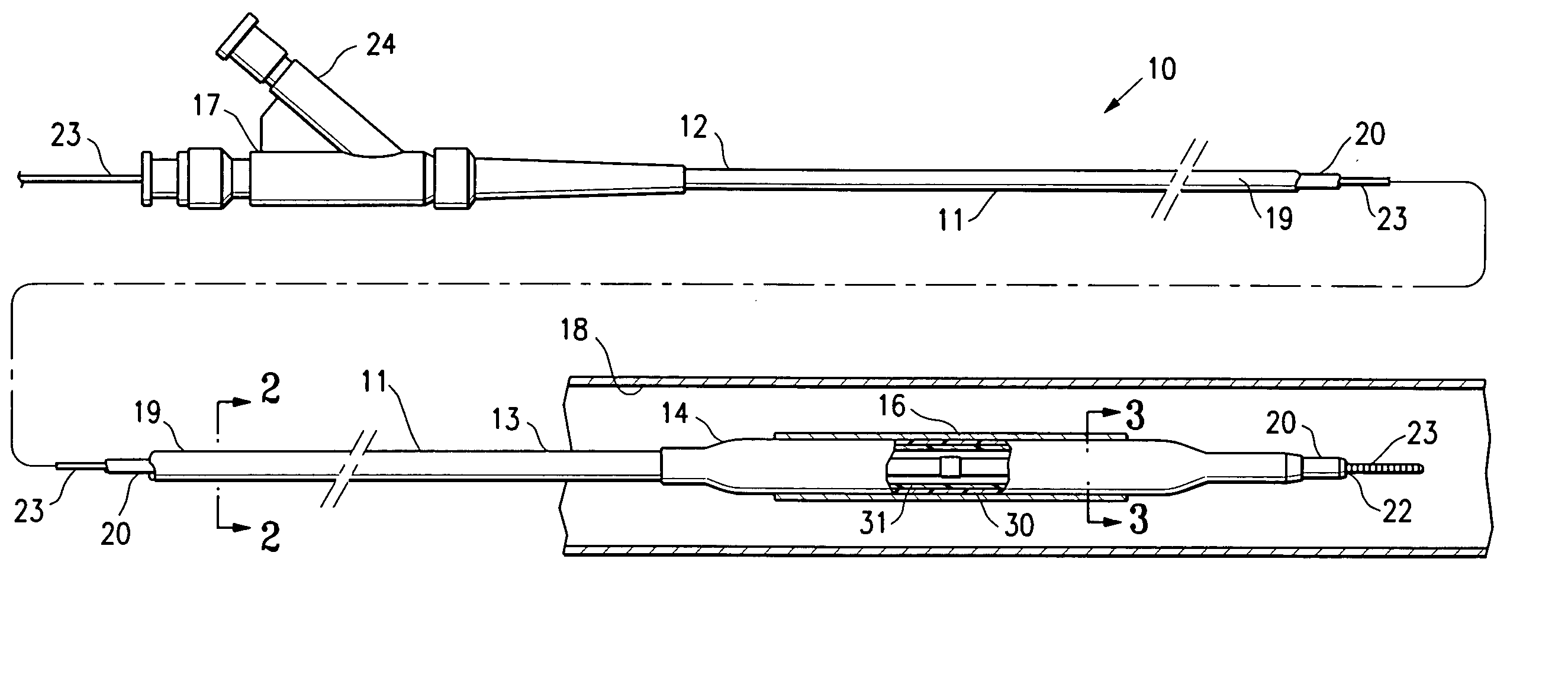
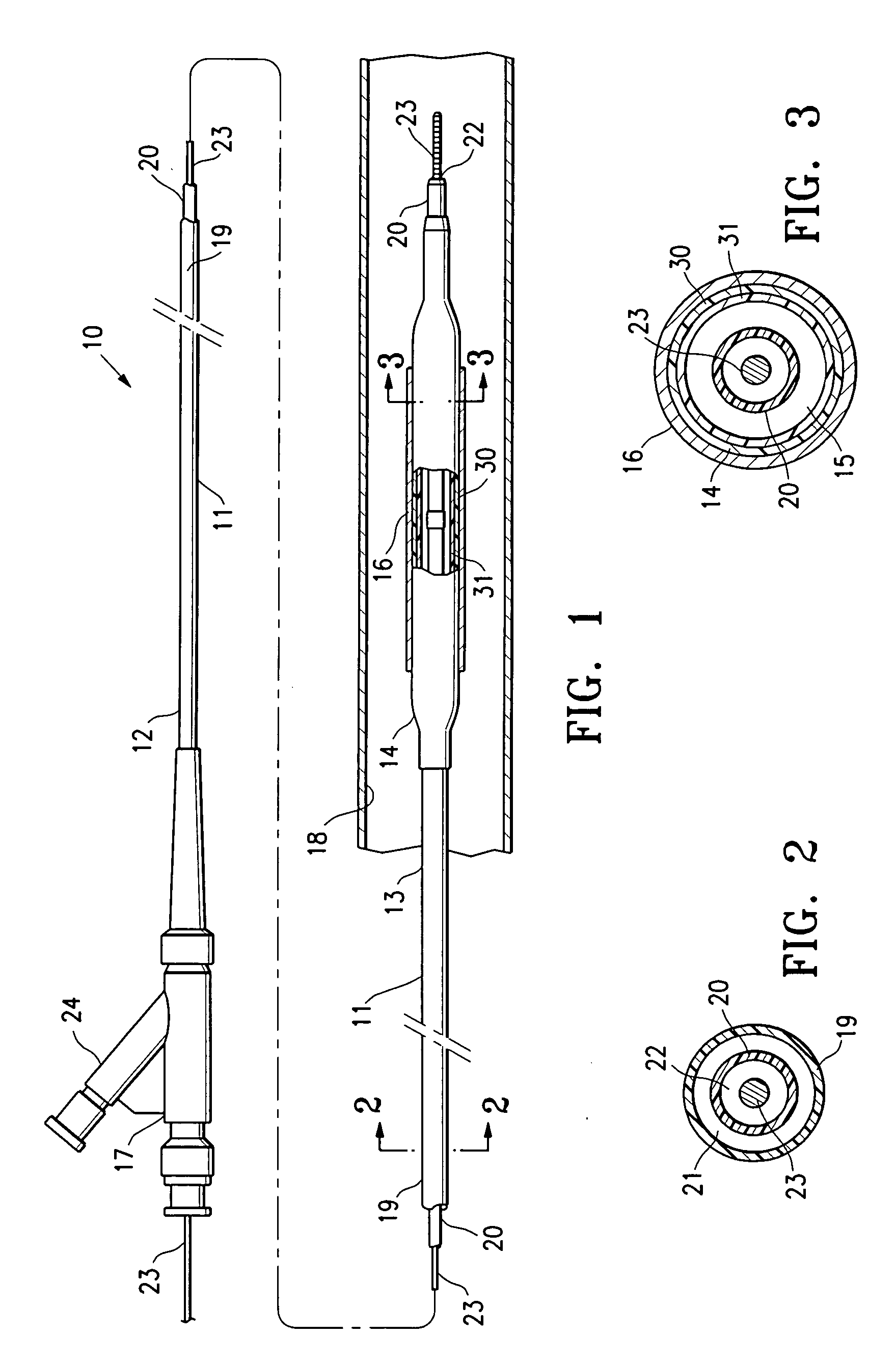
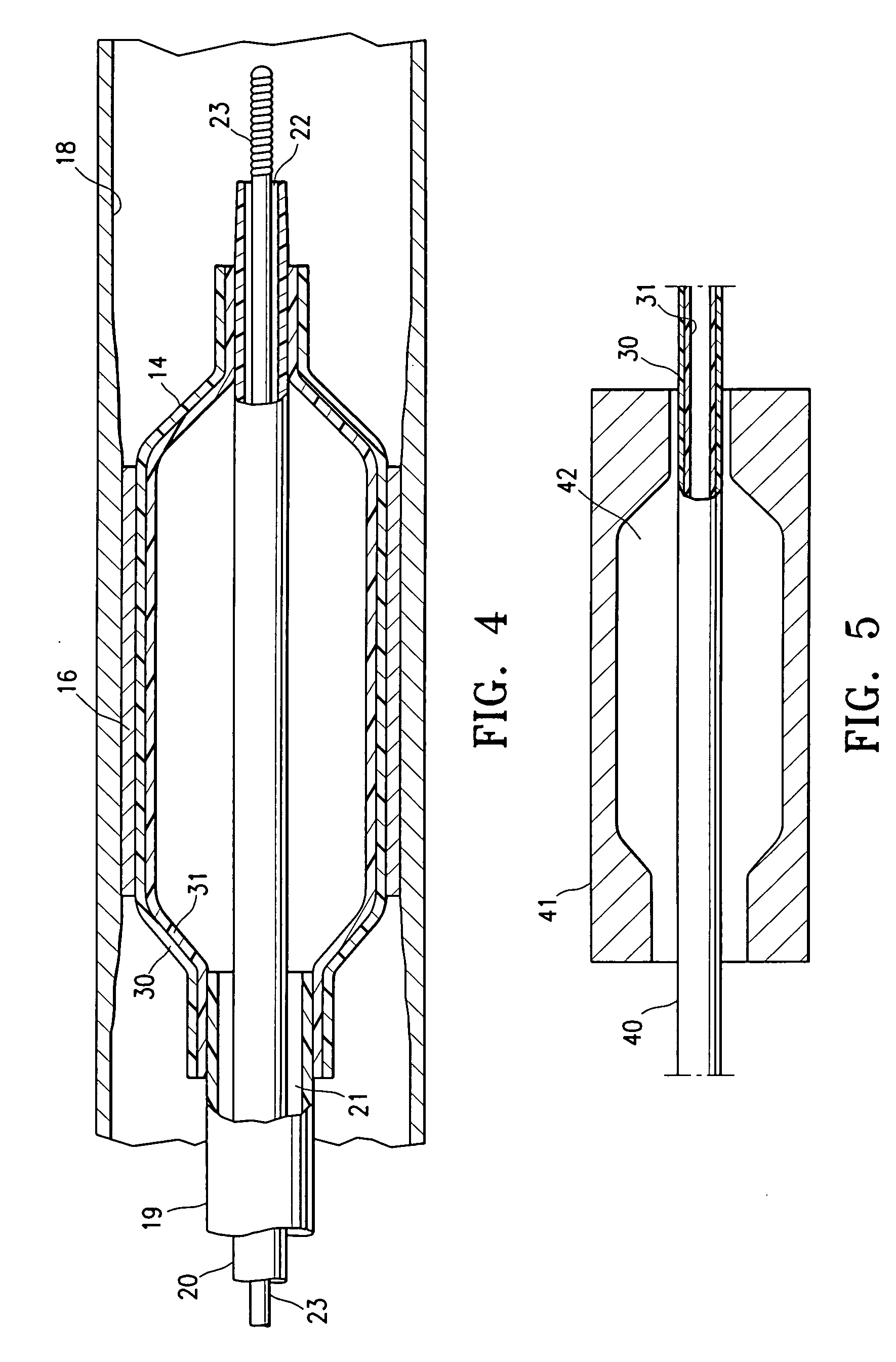
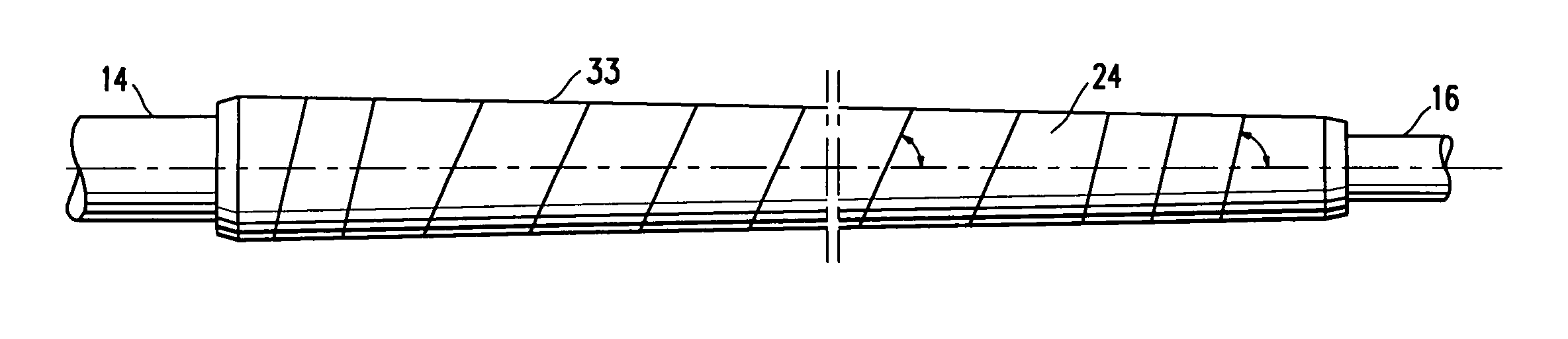
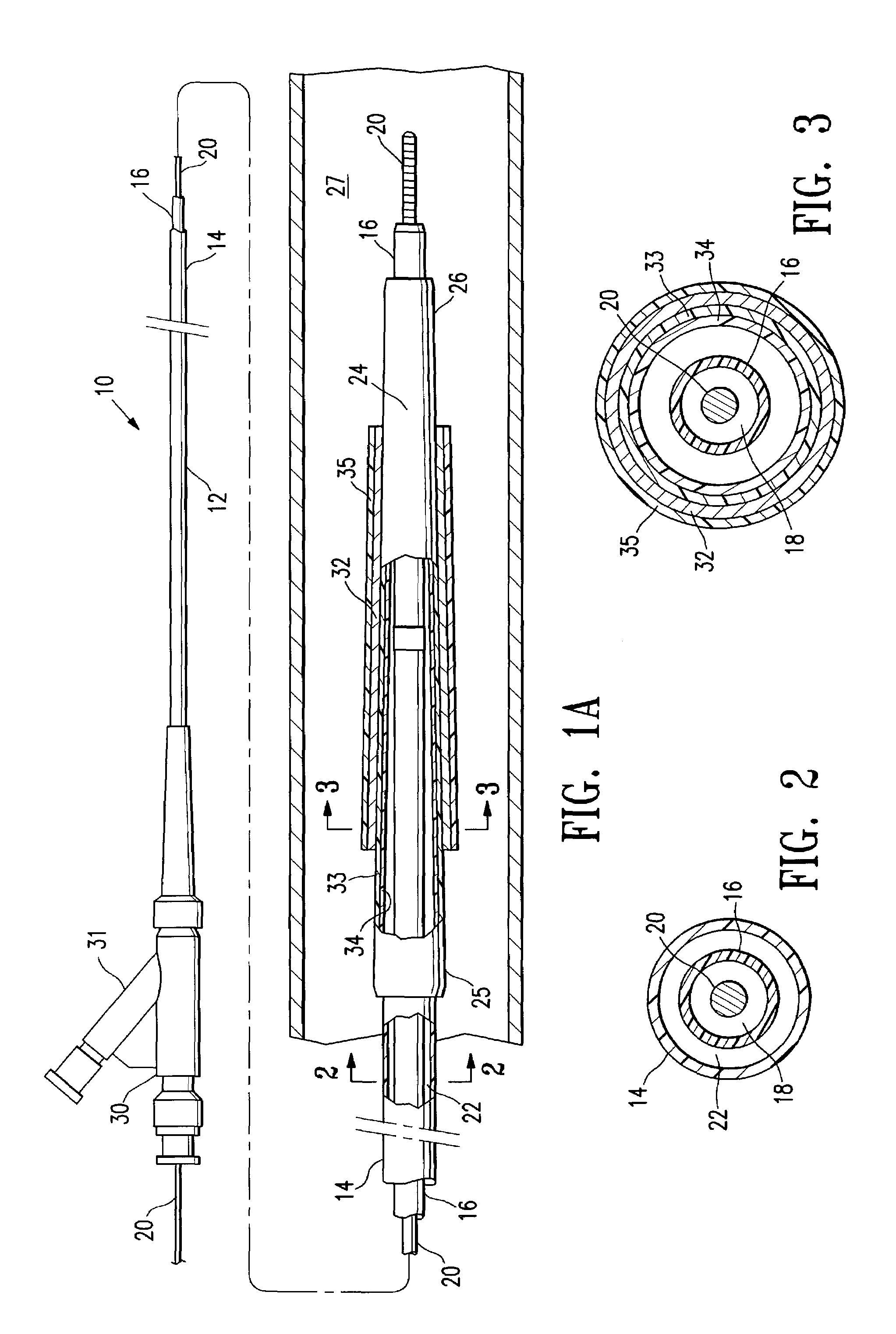
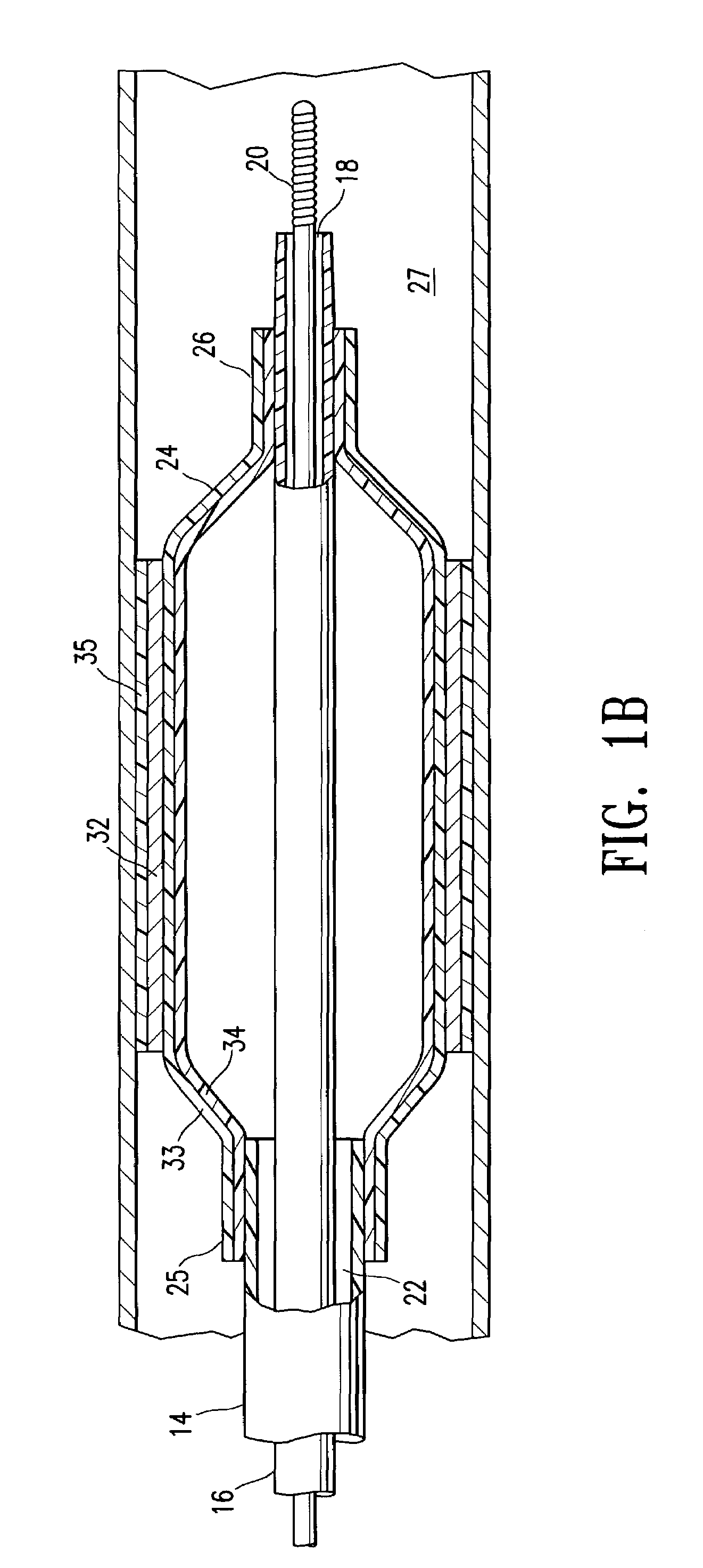
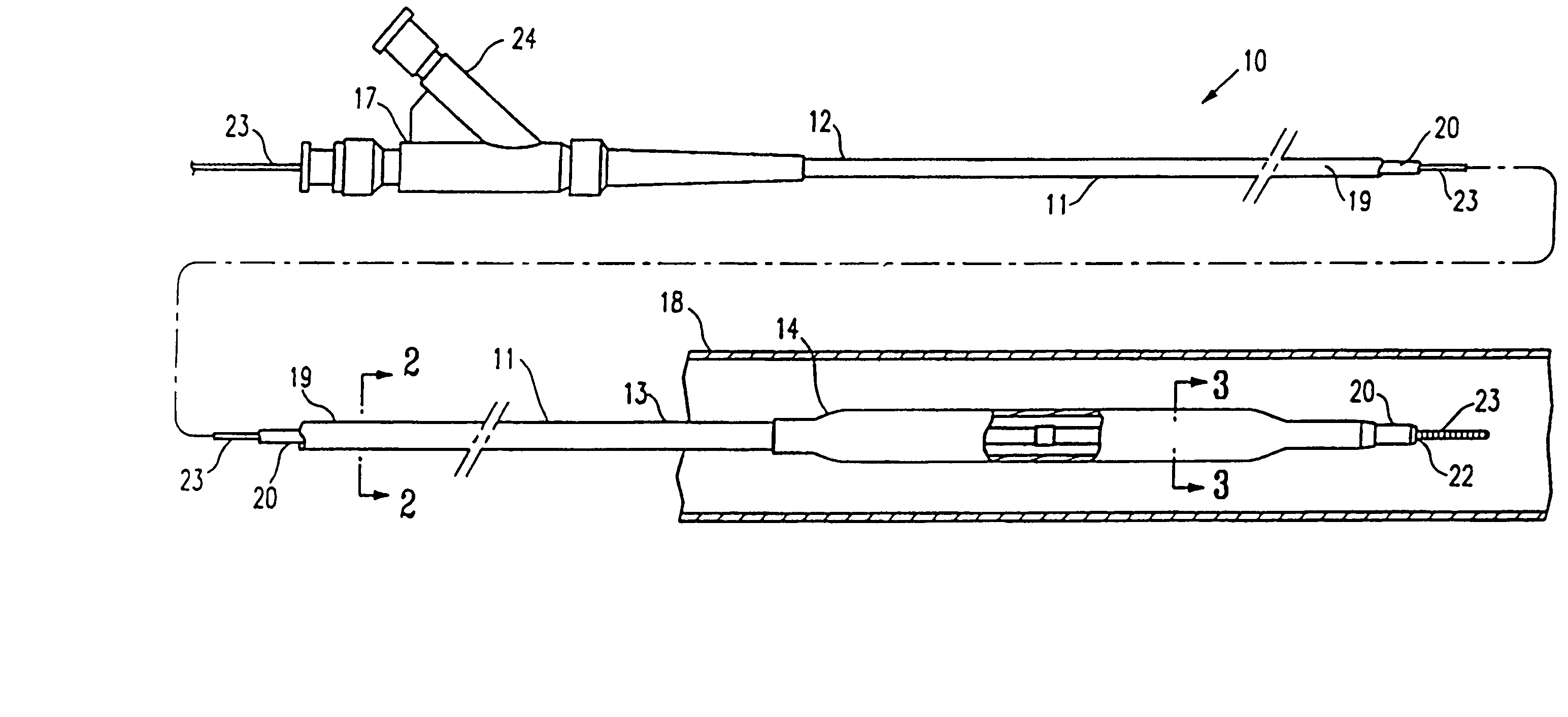
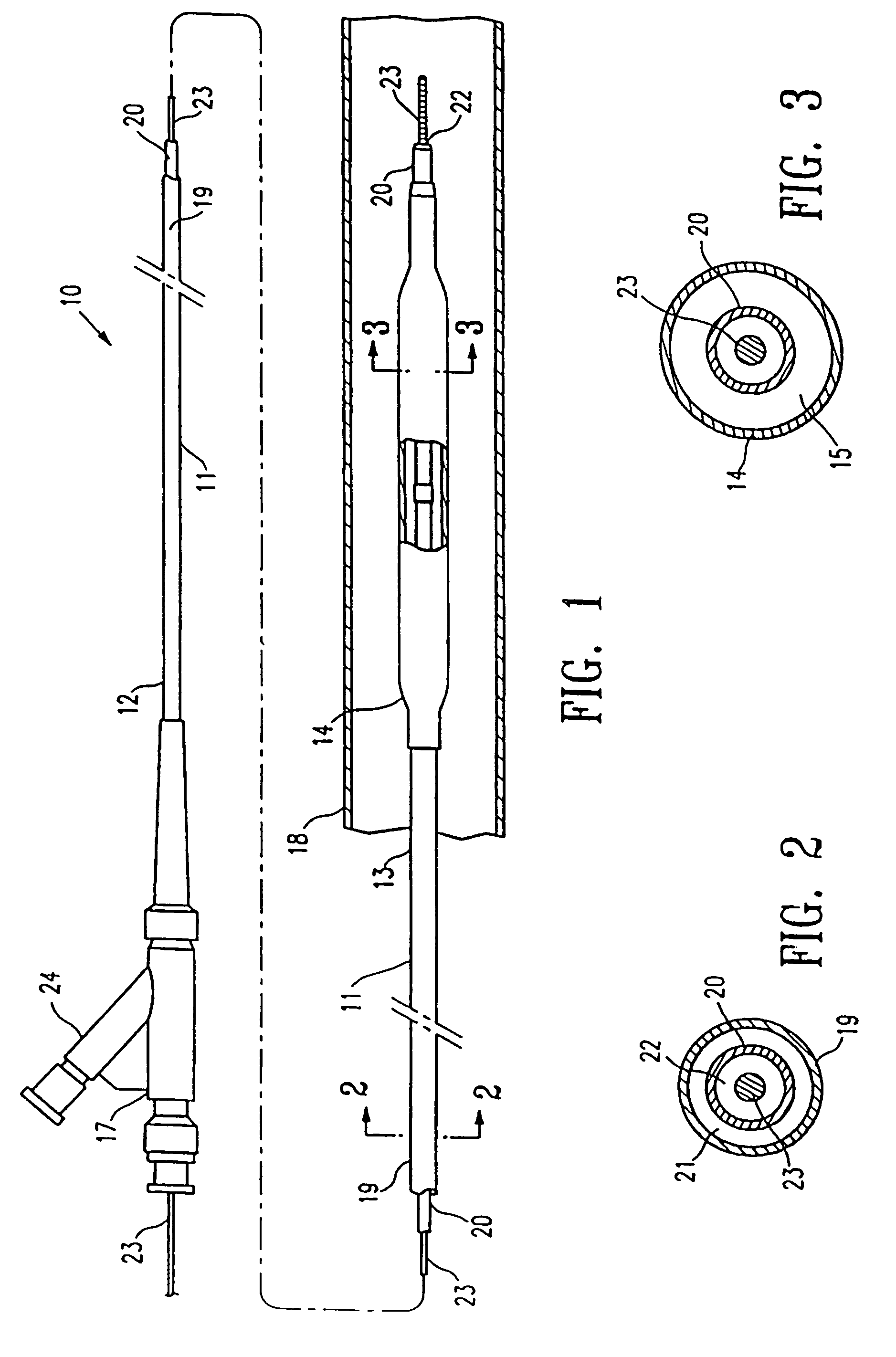
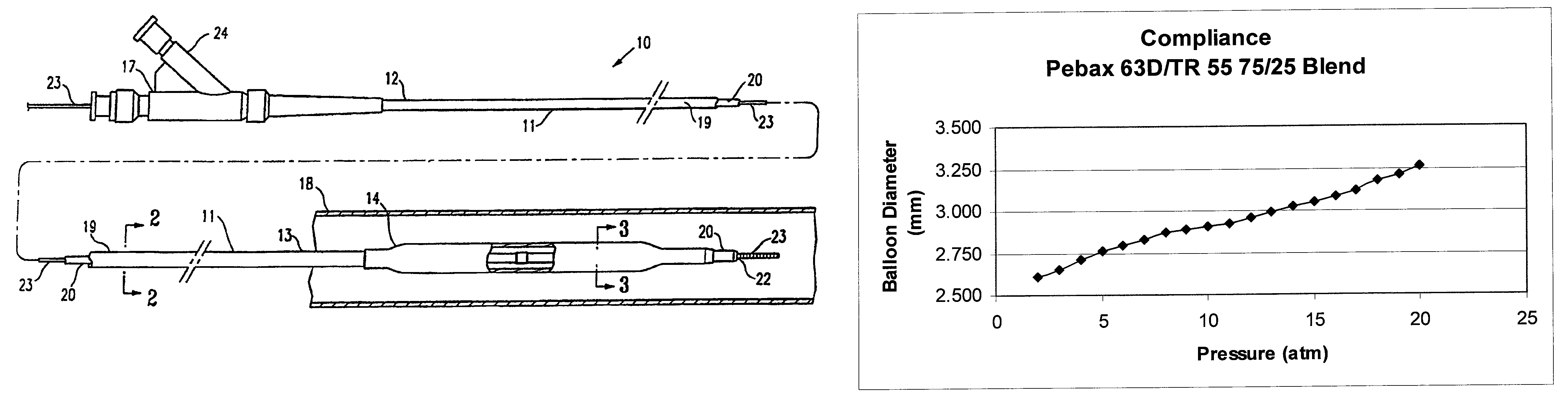
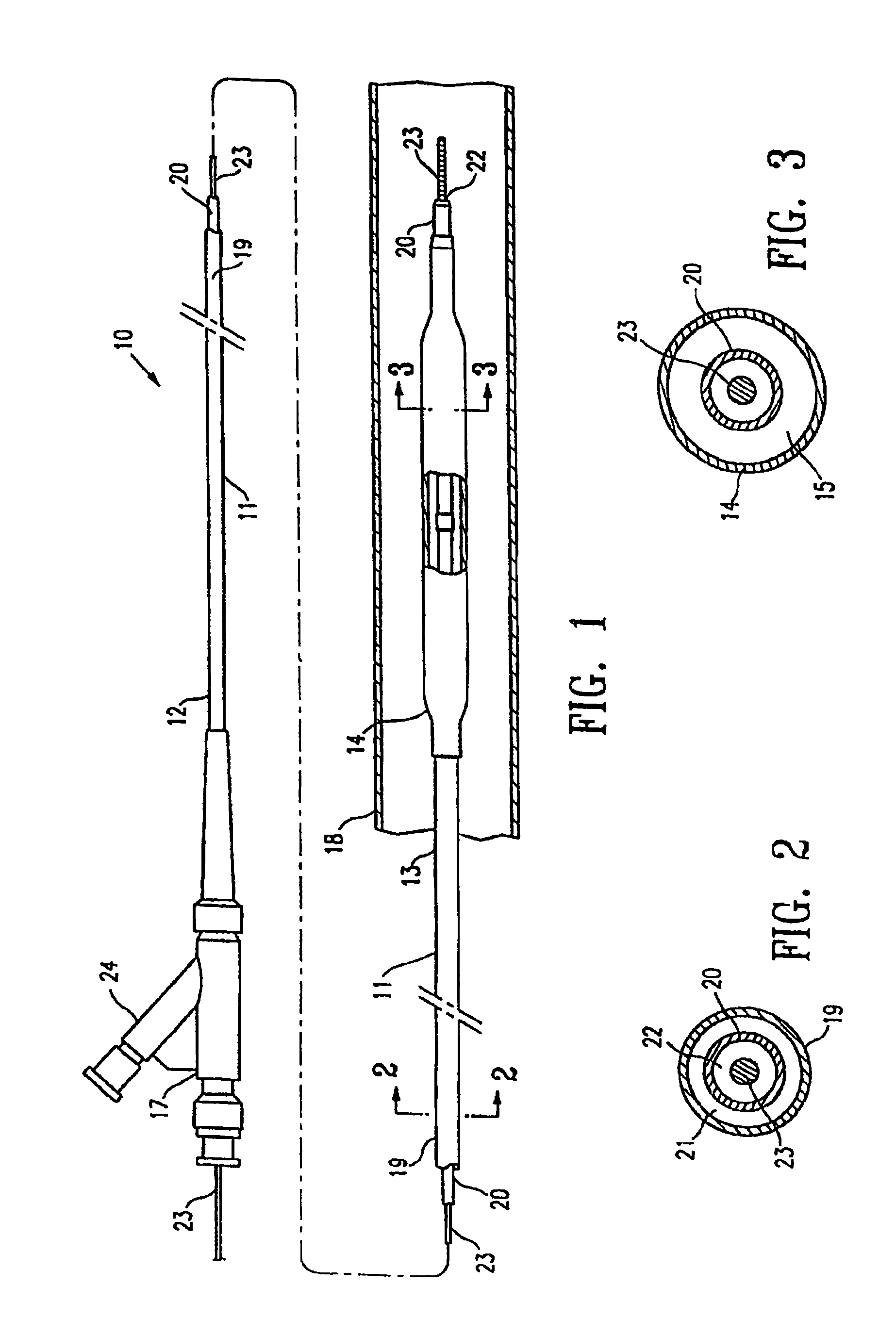
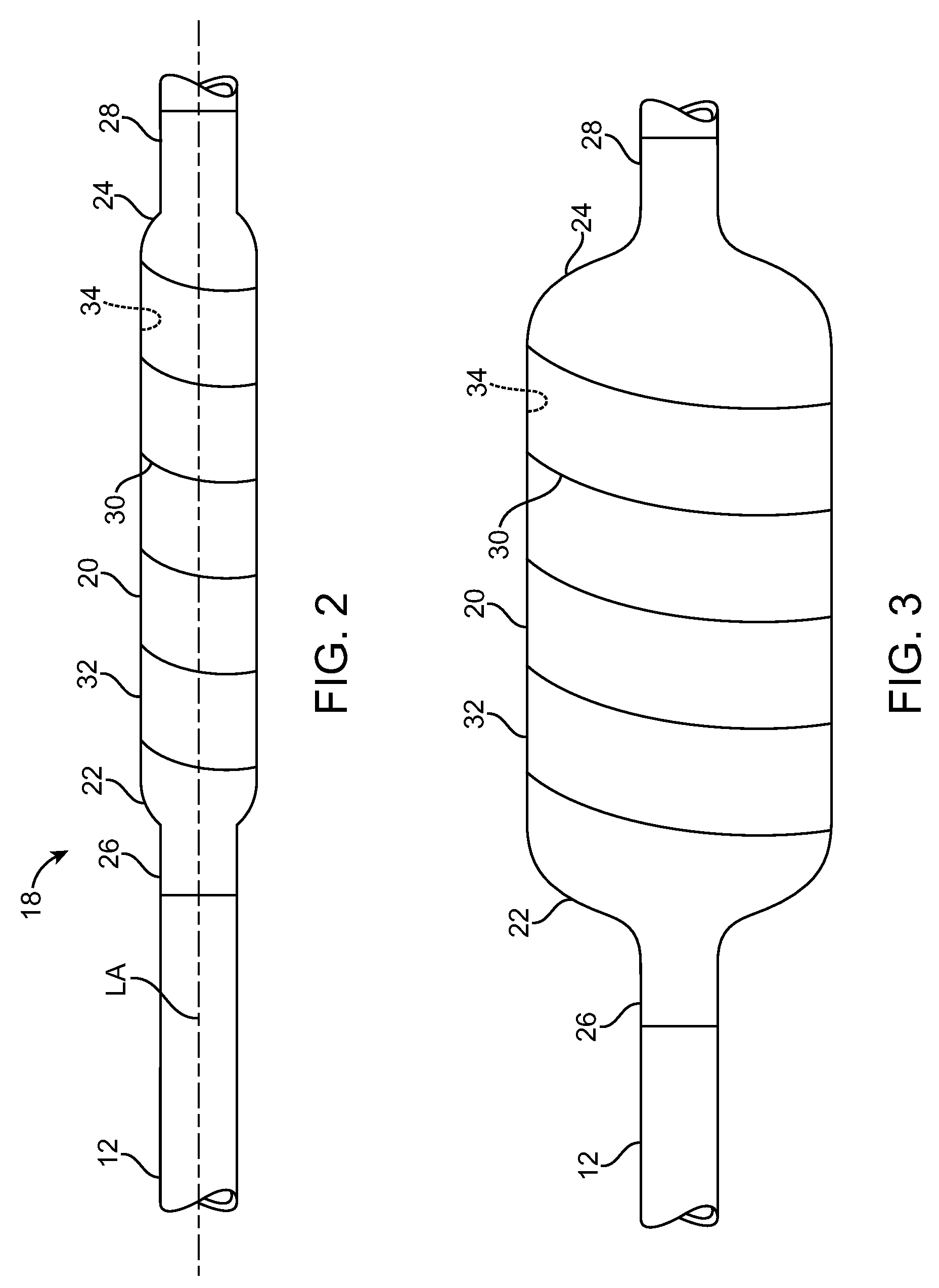
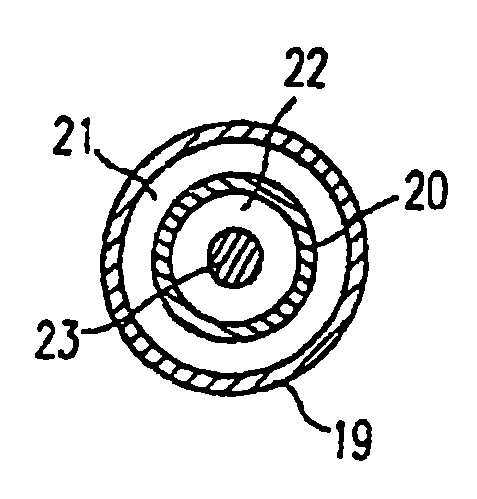
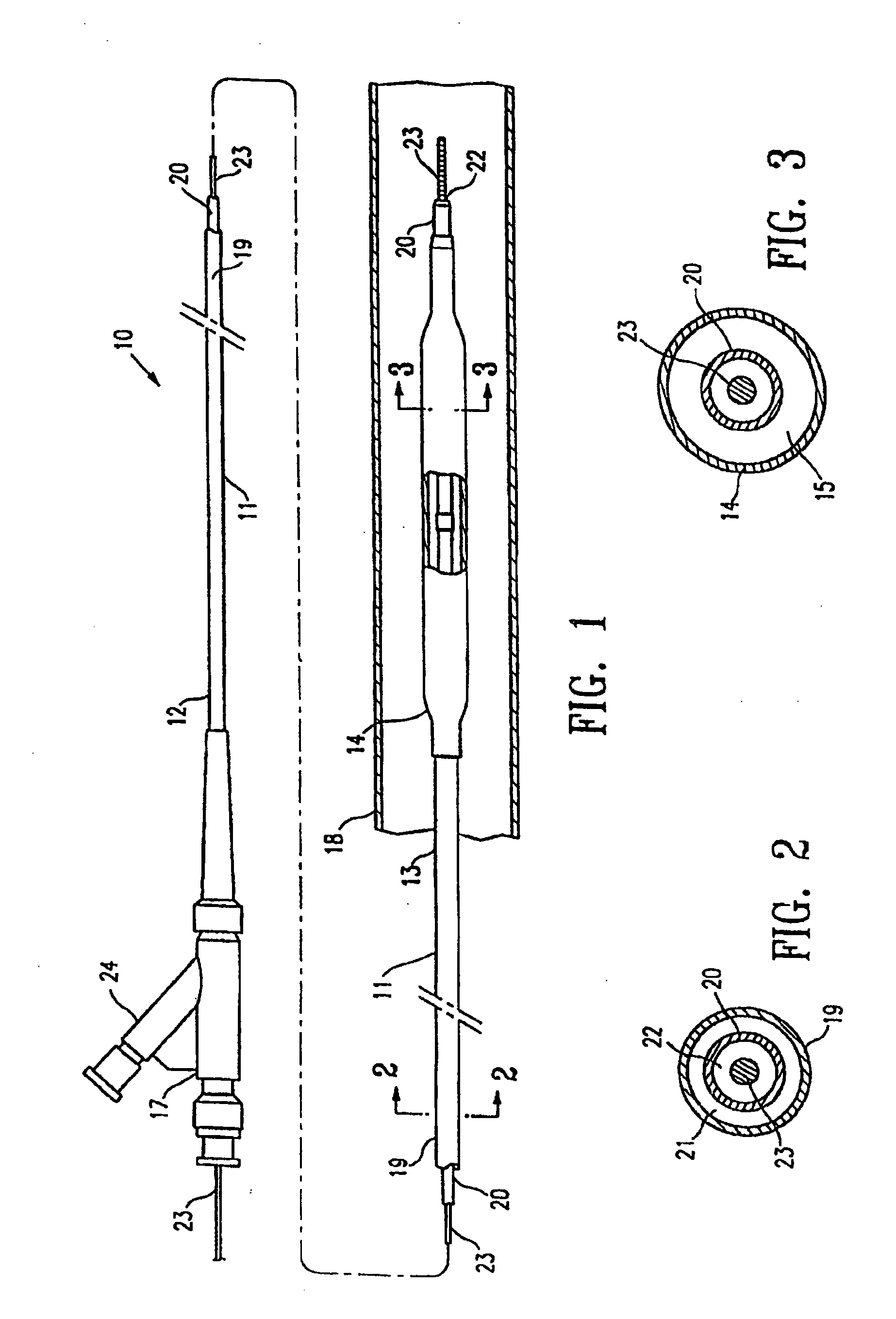
Non-compliant multilayered balloon for a catheter
ActiveUS7828766B2Improved low complianceIncrease flexibilityStentsBalloon catheterMedicineBalloon catheter
Owner:ABBOTT CARDIOVASCULAR
Non-compliant multilayered balloon for a catheter
ActiveUS20070142771A1Improved low complianceIncrease flexibilityStentsBalloon catheterMedicineBalloon catheter
A balloon catheter having a multi-layered balloon which has a first layer and at least a second layer, and which has noncompliant limited radial expansion beyond the nominal diameter of the balloon. By selecting the polymeric materials forming the balloon layers, and arranging and radially expanding the multiple layers of the balloon in accordance with the invention, a balloon is provided having an improved low compliance, preferably in combination with high flexibility and softness.
Owner:ABBOTT CARDIOVASCULAR
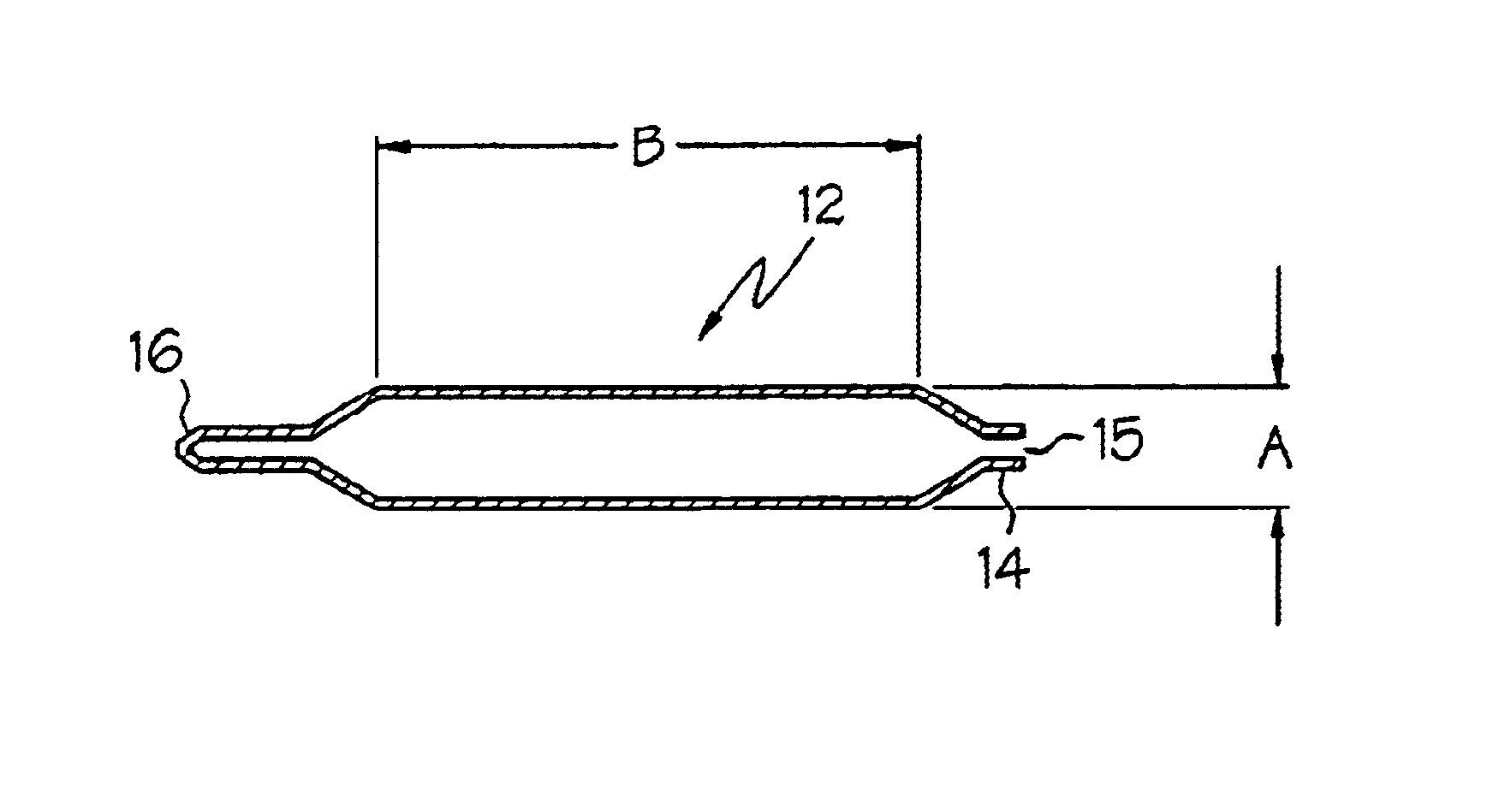
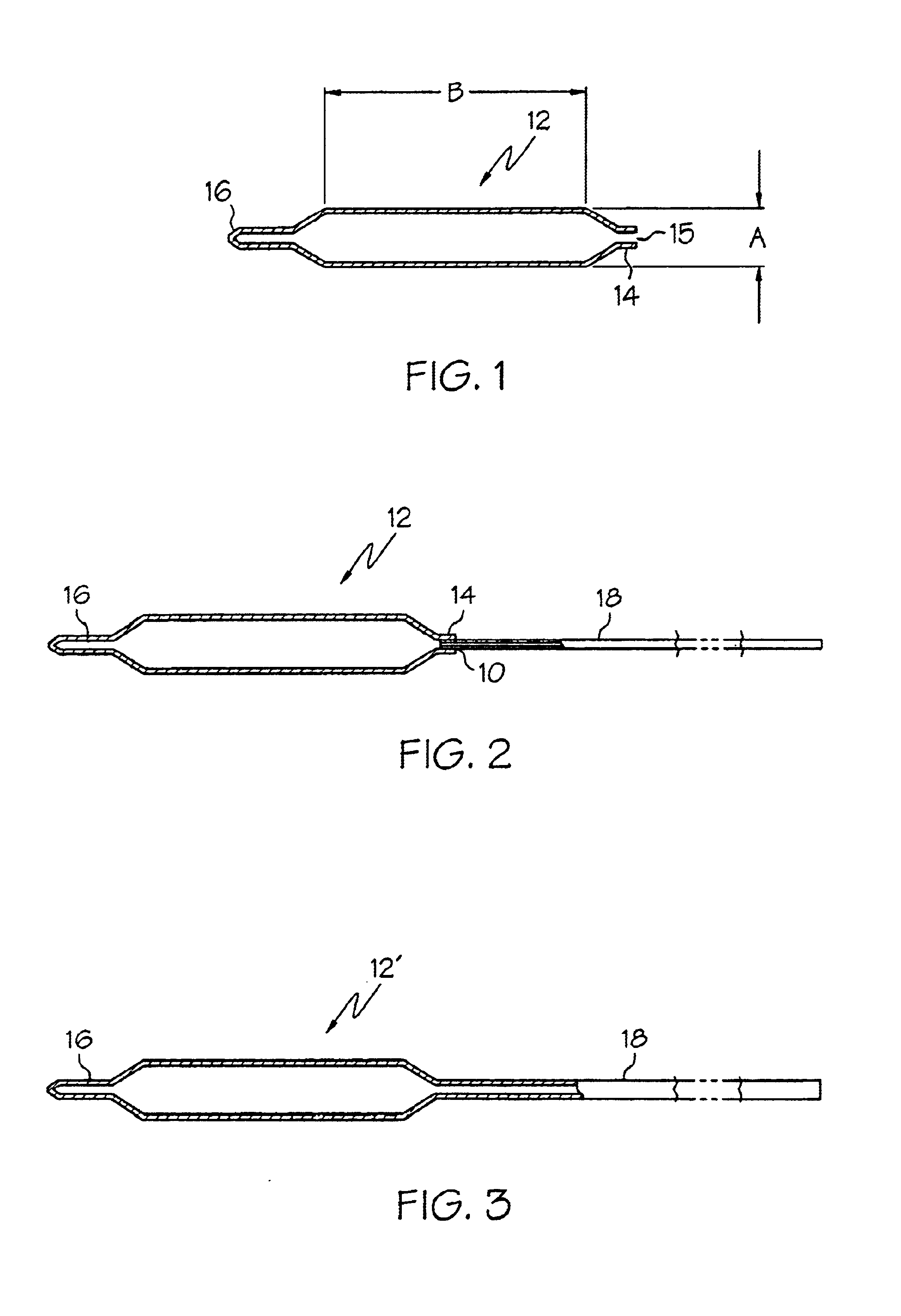
Catheter balloon
An expandable medical device or component thereof including a tubular body formed of a wrapped sheet of porous polymeric material fused together, the tubular body having a fused seam at an angle relative to the longitudinal axis of the tubular body which changes along the length of the tubular body from a first angle to a second angle greater than the first angle. The sheet of porous polymeric material is wound and then fused together such that the winding angle is less in a first longitudinal section of the tubular body compared with the winding angle in a second longitudinal section of the tubular body, in order to provide the second section with greater resistance to expansion (i.e., lower compliance) than the first section.
Owner:ABBOTT CARDIOVASCULAR
Medical device balloon
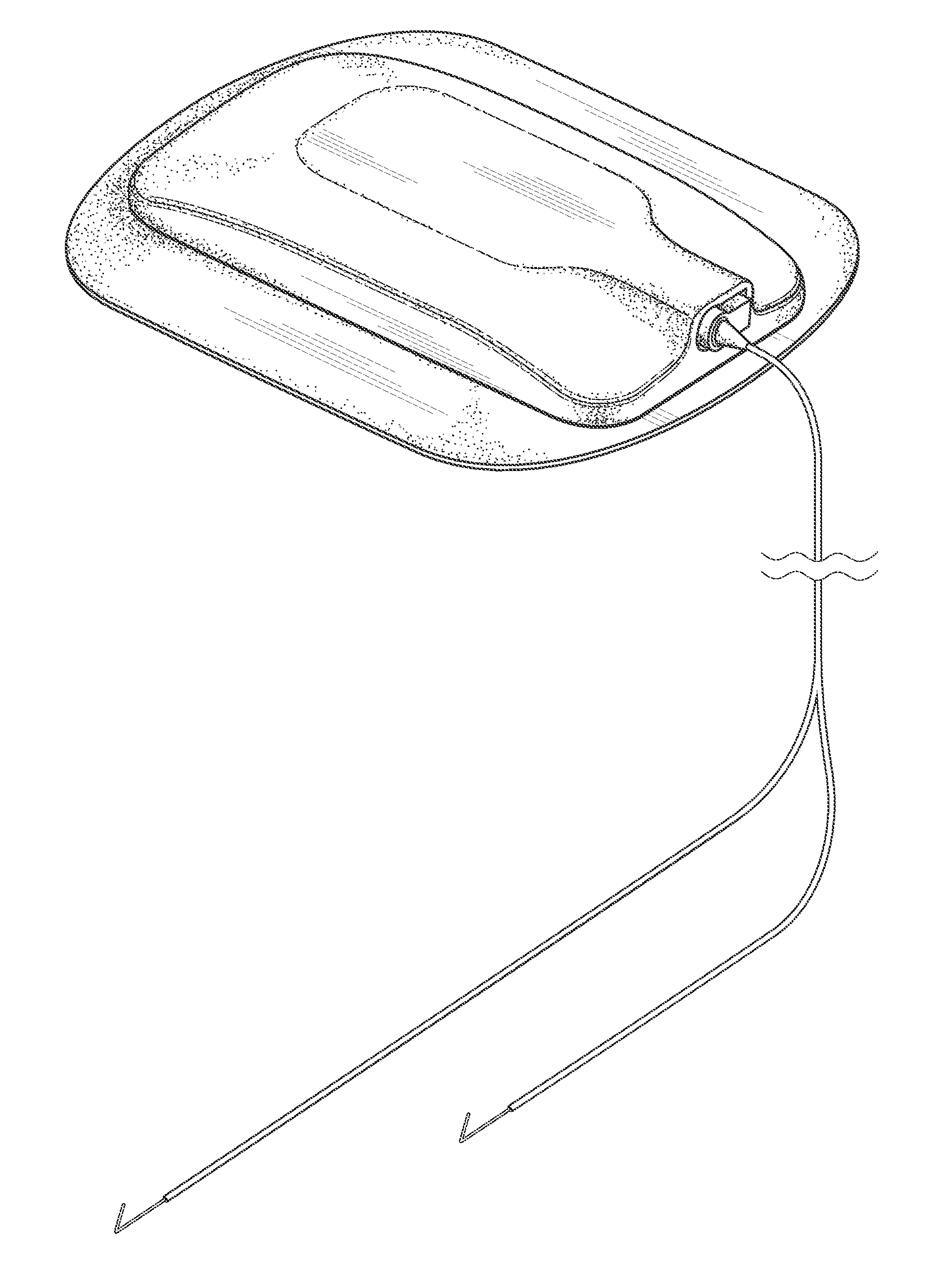
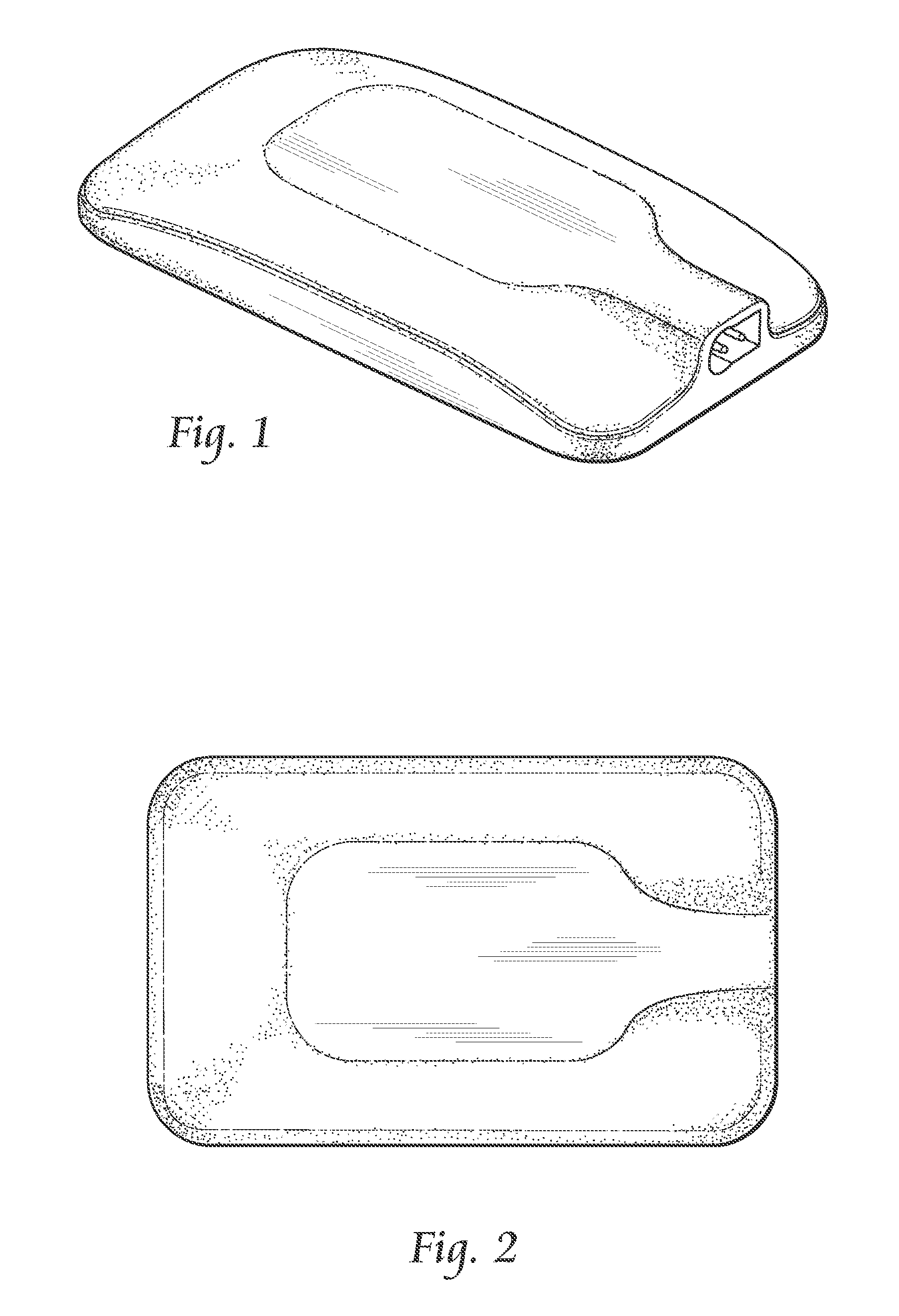
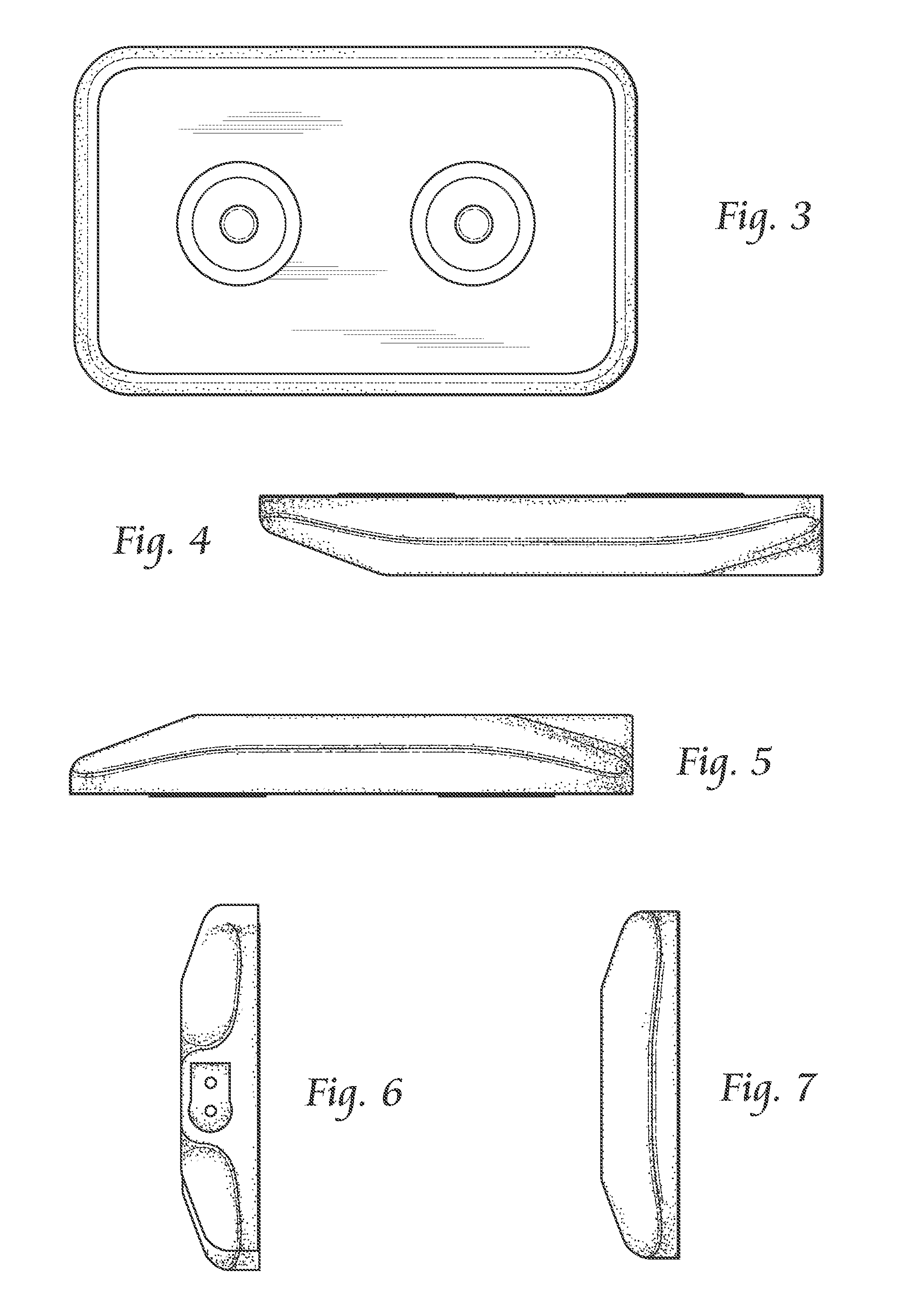
InactiveUS7074206B2Good flexibilityIncrease flexibilitySynthetic resin layered productsSurgeryMedicinePliability
A balloon catheter having a balloon formed at least in part of a blend of a first polymeric material having a first Shore durometer hardness, and at least a second polymeric material having a second Shore durometer hardness less than the Shore durometer hardness of the first polymeric material. The balloon of the invention has enhanced softness and flexibility due to the presence of the second polymeric material, and a lower than expected compliance. In a presently preferred embodiment, the balloon is formed of a blend of polymeric materials comprising polyether block amides.
Owner:ABBOTT CARDIOVASCULAR
Systems and methods for providing percutaneous electrical stimulation
Systems and methods according to the present invention relate to a novel peripheral nerve stimulation system for the treatment of pain, such as pain that exists after amputation.
Owner:SPR THERAPEUTICS
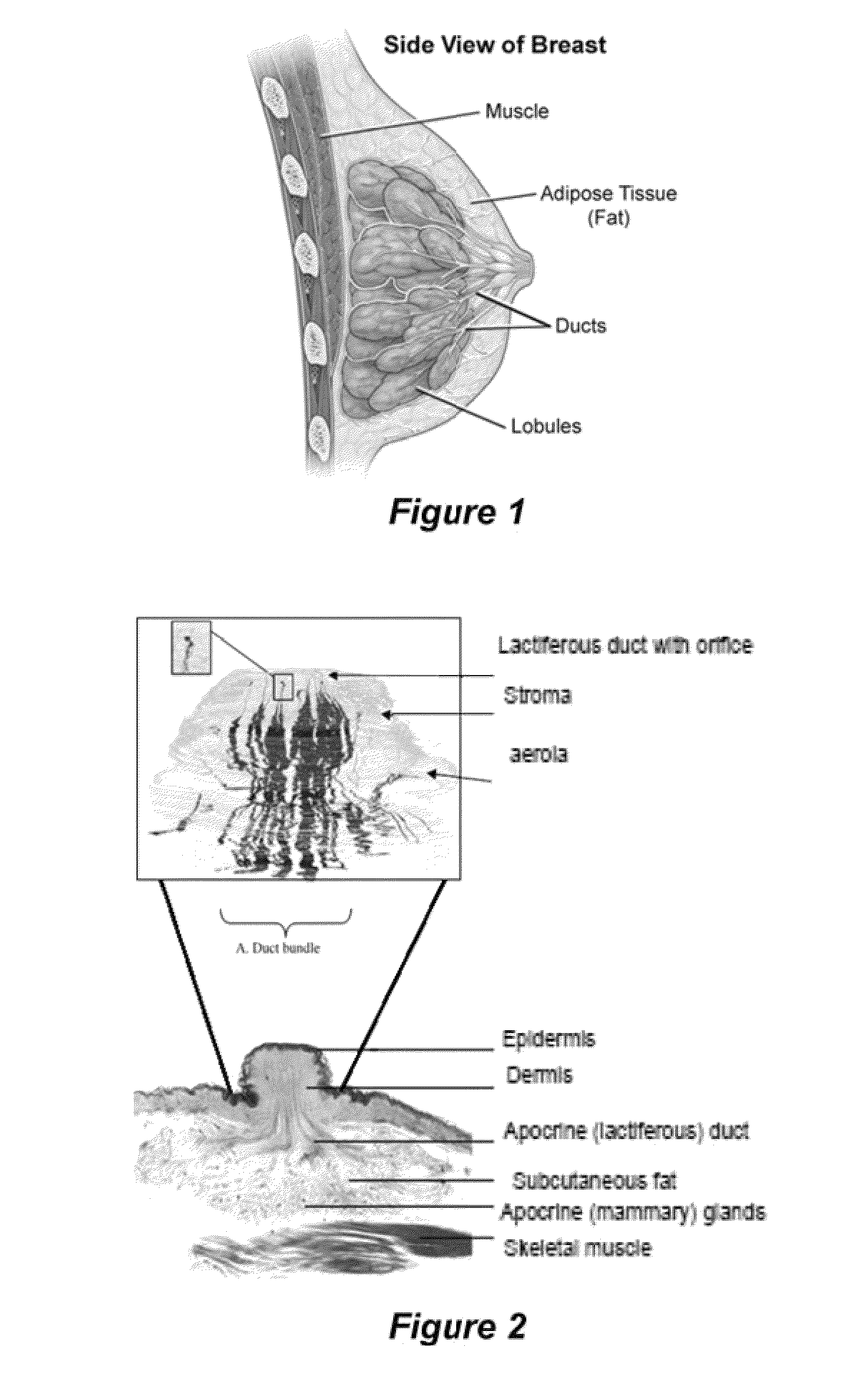
Compositions and Methods for Localized Drug Delivery through Mammary Papillae
ActiveUS20140088059A1Improve throughputEasy to keepOrganic active ingredientsBiocideDiagnostic agentAreola
The invention provides compositions and methods for the prevention, diagnosis, or treatment of conditions affecting breast tissue. The compositions can include one or more therapeutic agents or diagnostic agents, and an effective carrier. The composition can be specifically adapted for transdermal permeation through the mammary papilla, areola, or a combination thereof, and into underlying breast tissue.
Owner:SOUTH DAKOTA STATE UNIVERSITY
Drug-containing patch
ActiveUS7744918B2Low melting pointImprove skinPharmaceutical non-active ingredientsAbsorbent padsMedicineSurgery
The problem of the invention is to provide a drug-containing patch which is very favorable in percutaneous absorbability and is excellent in sustainability of the drug efficacy, that is, the drug-containing patch having a sufficient percutaneous absorbability and effect sustainability in a degree to be actually used for therapy of patients. The problem is solved by a patch comprising a drug, a melting point lowering agent and an adhesive base.
Owner:HISAMITSU PHARM CO INC
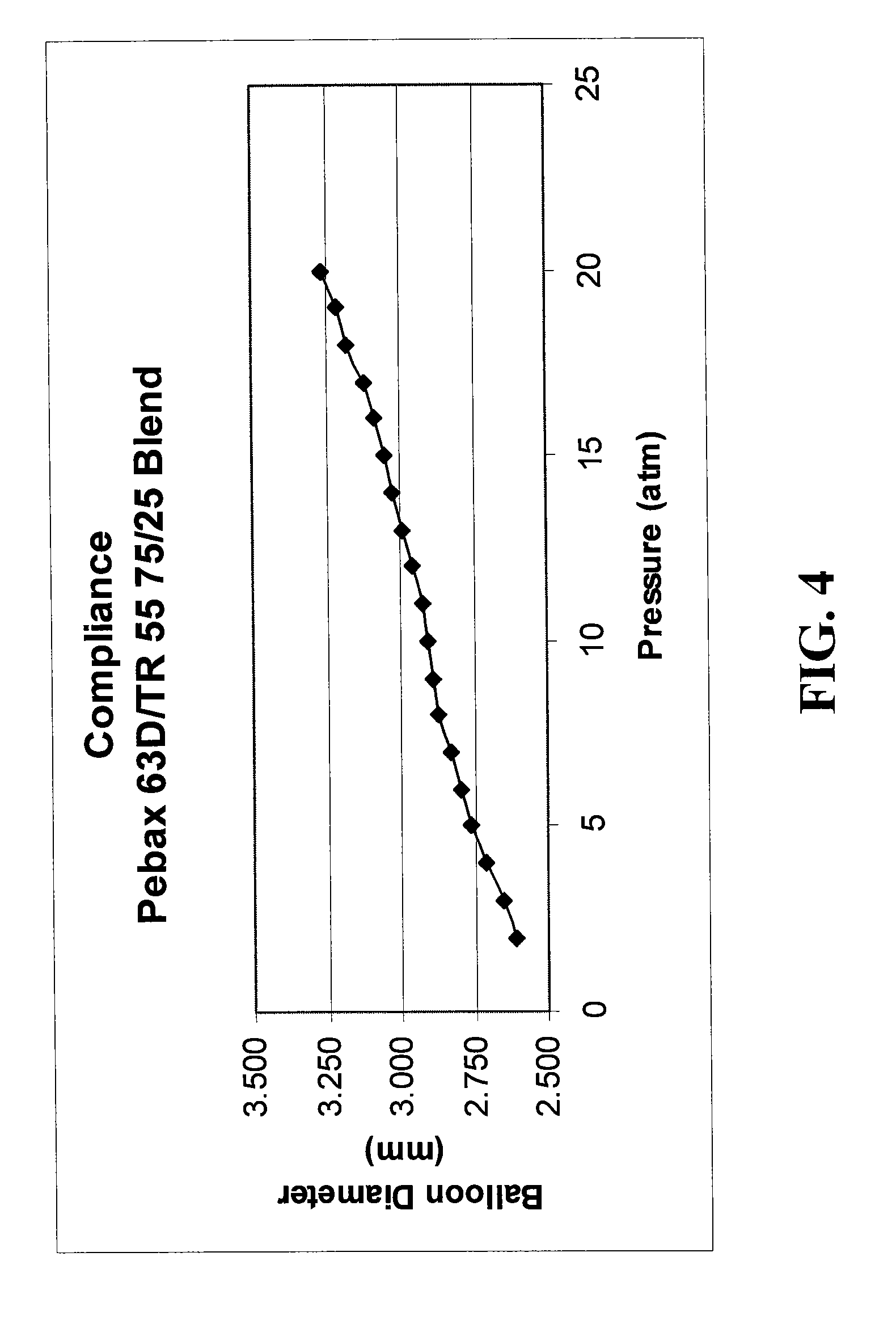
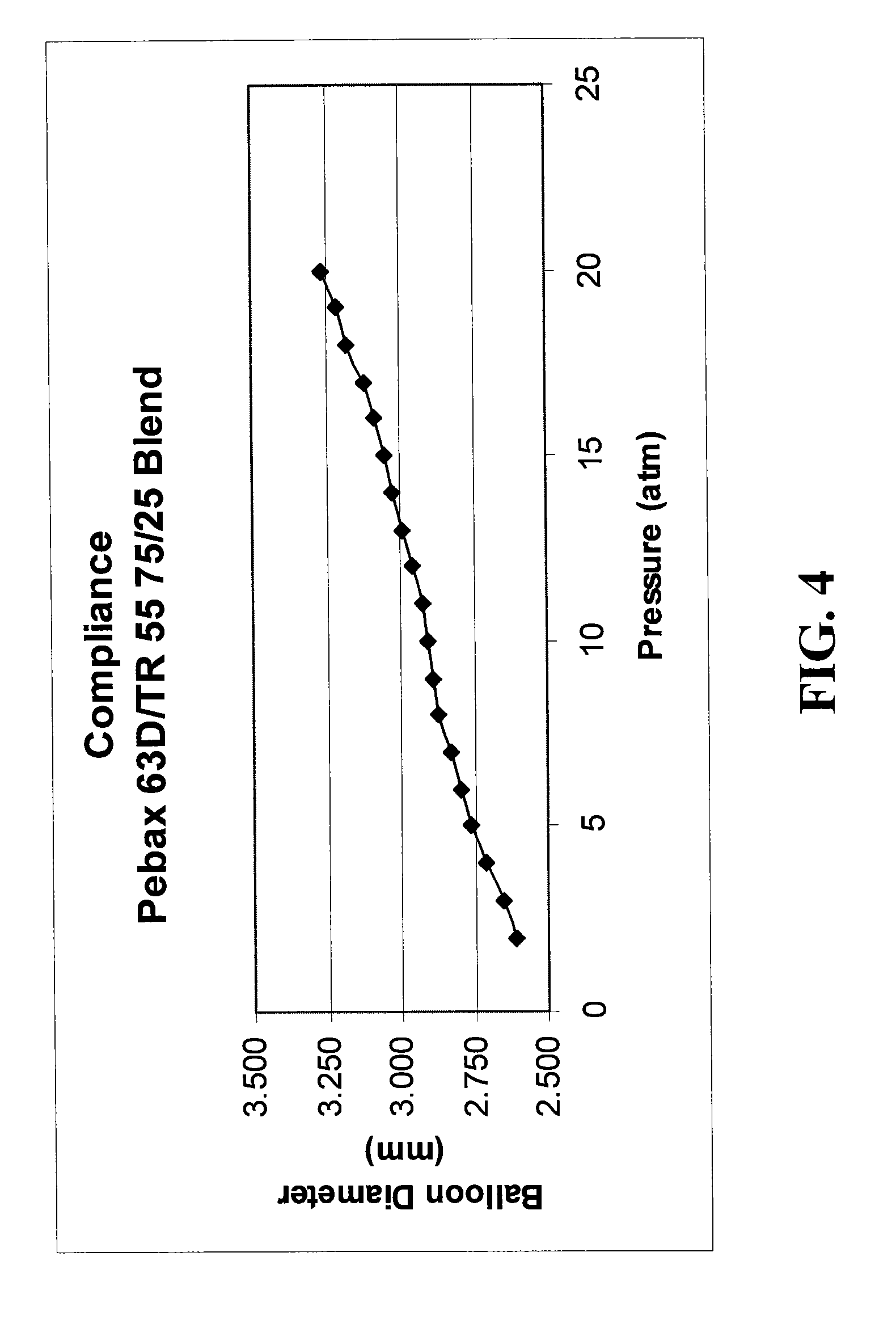
Low compliant catheter tubing
ActiveUS8070719B2Improve complianceImproves strength and modulusStentsSurgeryPolyamideBalloon catheter
Balloon catheter comprises a shaft having a proximal end, a distal end, and an inflation lumen extending therein, and a balloon on the shaft which has an interior in fluid communication with the inflation lumen. The balloon is formed of a blend of polymeric materials comprising a transparent amorphous nylon having a Shore D duromcter hardness of not less than 77D and being not more than about 40% by weight of the blend, and a polyamide or a polyether block amide having a Shore D duromcter hardness of no more than 73D. An increase in radial diameter of the balloon above nominal pressure for one atmosphere of pressure is no greater than 0.025 mm / atm. A guidewire catheter is also provided.
Owner:ABBOTT CARDIOVASCULAR
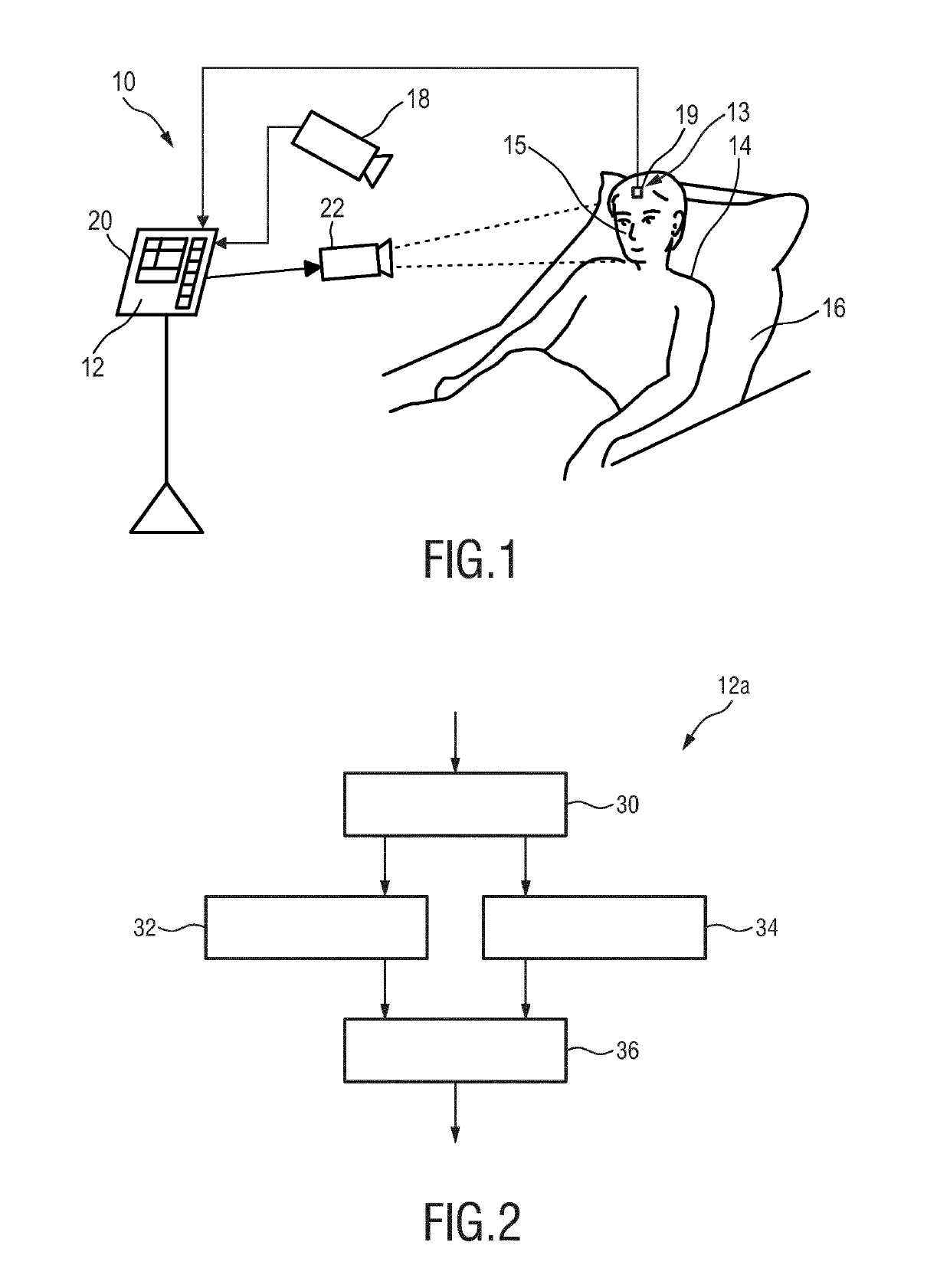
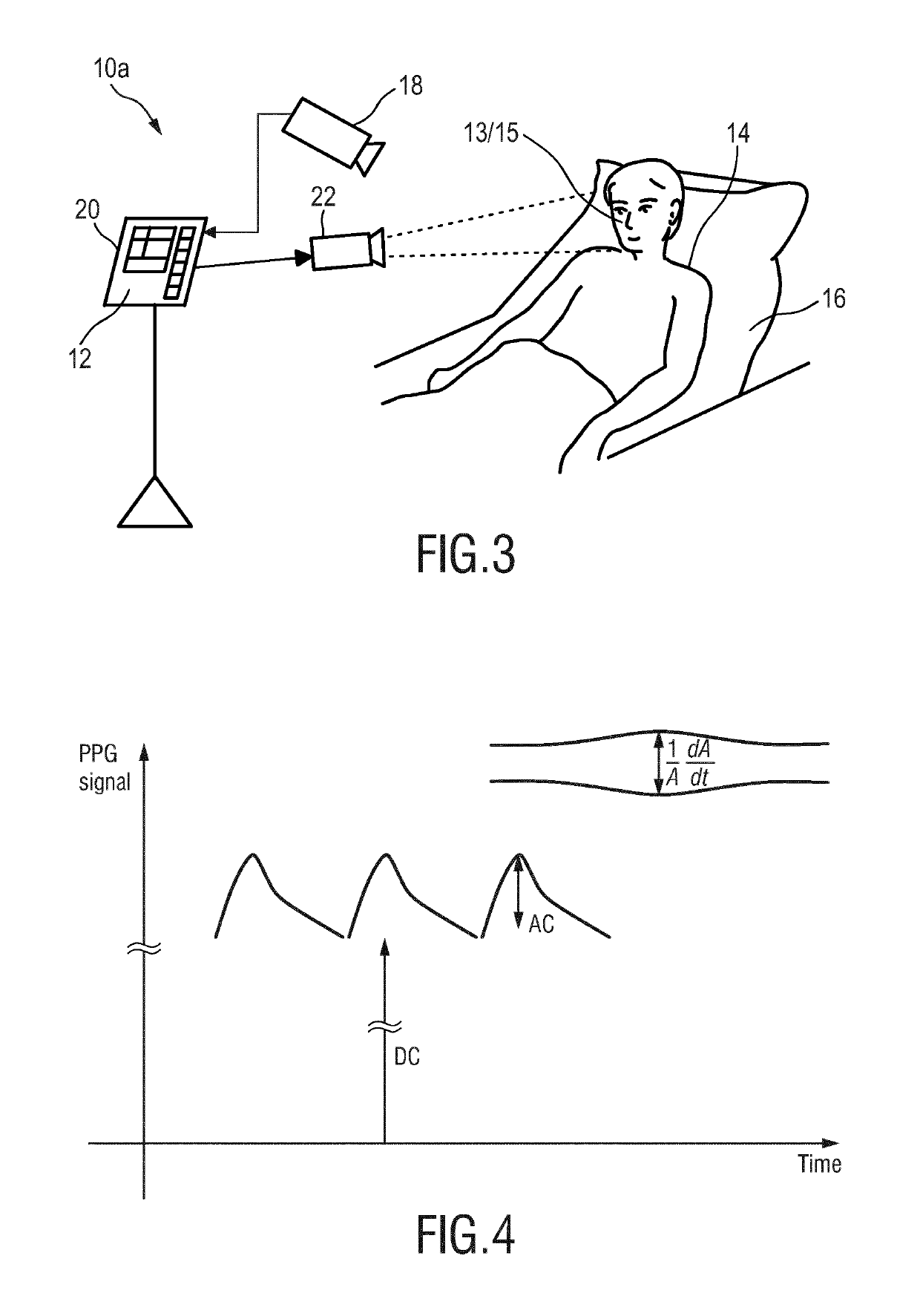
Device, system and method for monitoring of peripheral arterial perfusion of a subject
PendingUS20190175030A1Reduce pulsationLow complianceCatheterOptical sensorsNuclear medicineBlood vessel
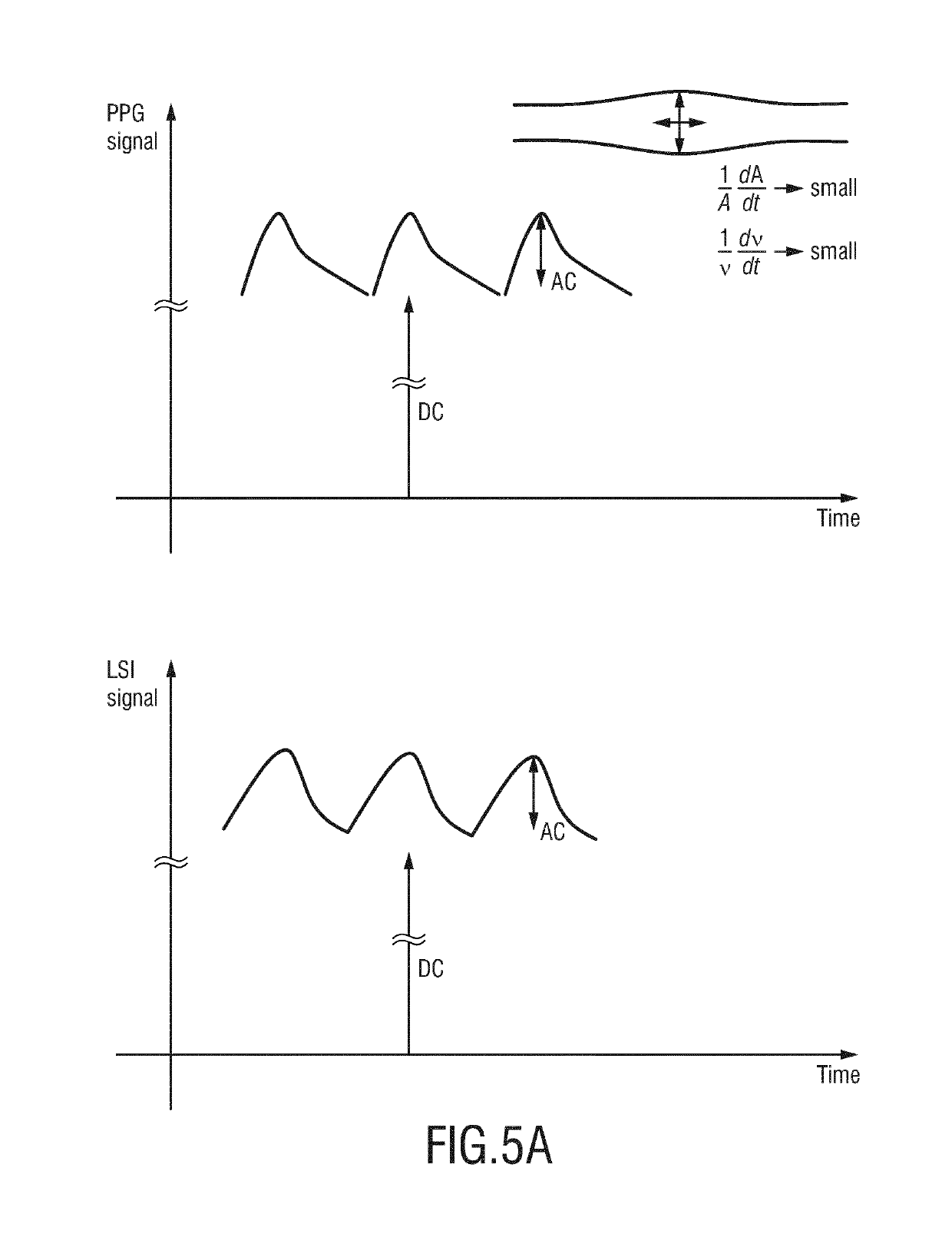
The present invention relates to a device, system and method for monitoring of peripheral arterial perfusion of a subject. To enable distinguishing between low pulsatility caused by centralization or low cardiac output, the proposed device comprises an input (30) for receiving first detection data of a tissue region of a subject, said first detection data being acquired over time by detecting radiation reflected from and / or transmitted through tissue of the subject, and for receiving second detection data of a skin region of subject, said second detection data being acquired over time by detecting radiation received from said tissue region in response to coherent light being emitted towards said skin region, a PPG unit (32) for deriving a photoplethysmography, PPG, signal from said first detection data, a flow unit (34) for deriving, from said second detection data, a flow signal indicative of a flow of light scattering particles within the skin region, and an evaluation unit (36) for evaluating said PPG signal and said flow signal to obtain information on the peripheral arterial perfusion, wherein the evaluation unit is adapted to determine a state of low vascular compliance and / or a state of low cardiac output based on a combined evaluation of said PPG signal and said flow signal.
Owner:KONINKLJIJKE PHILIPS NV
Viscous formulations and their use in needle-free injection
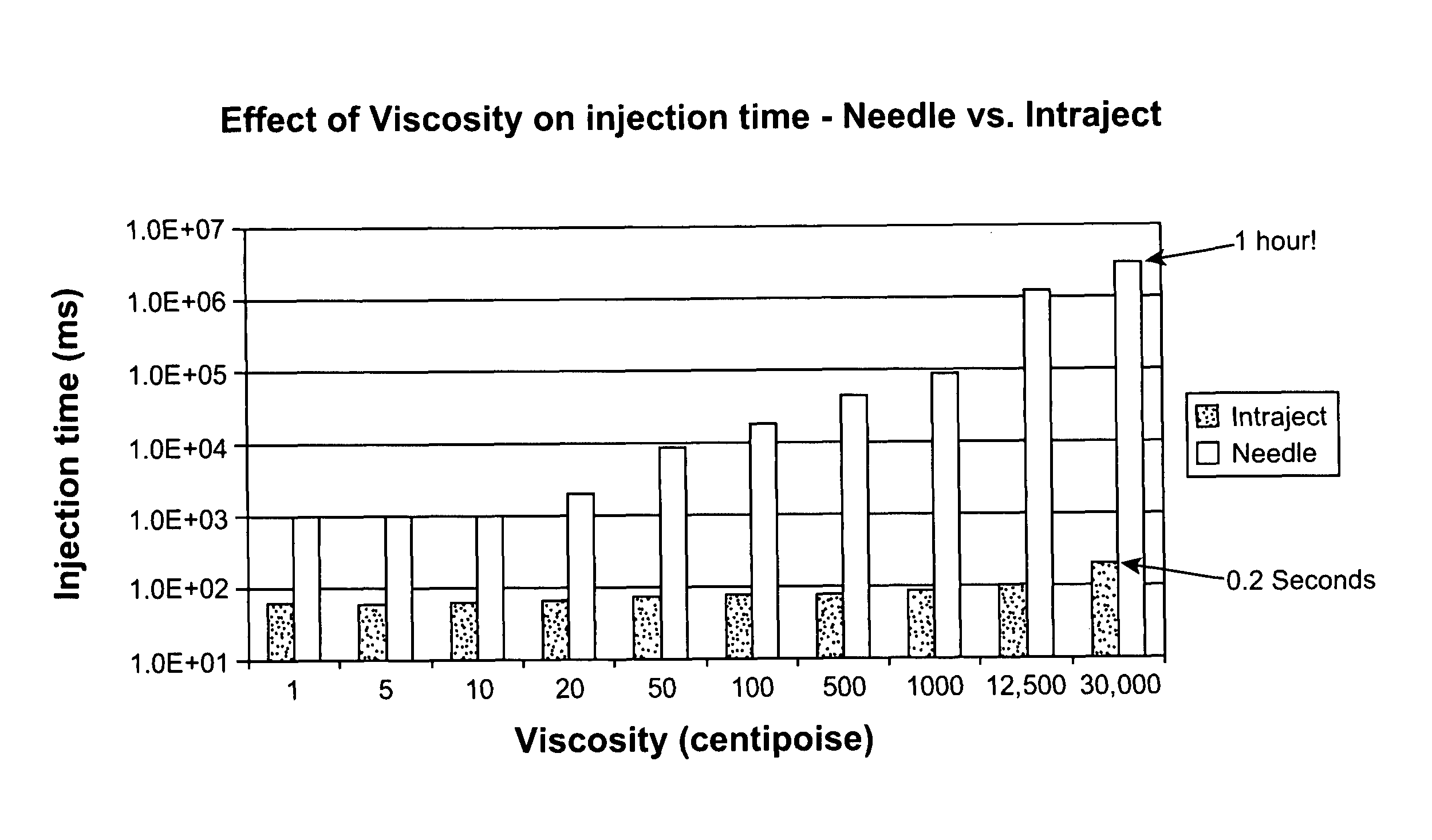
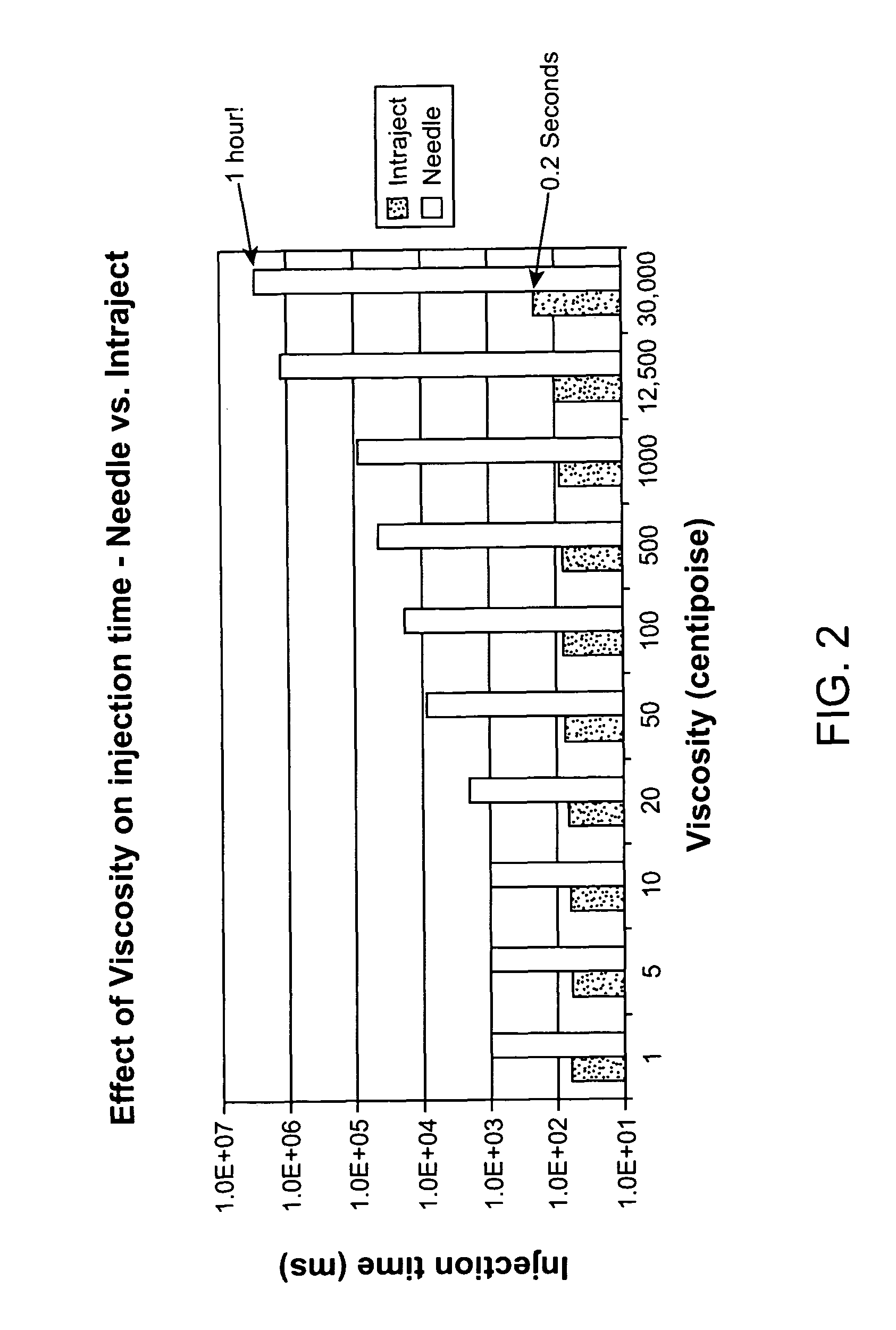
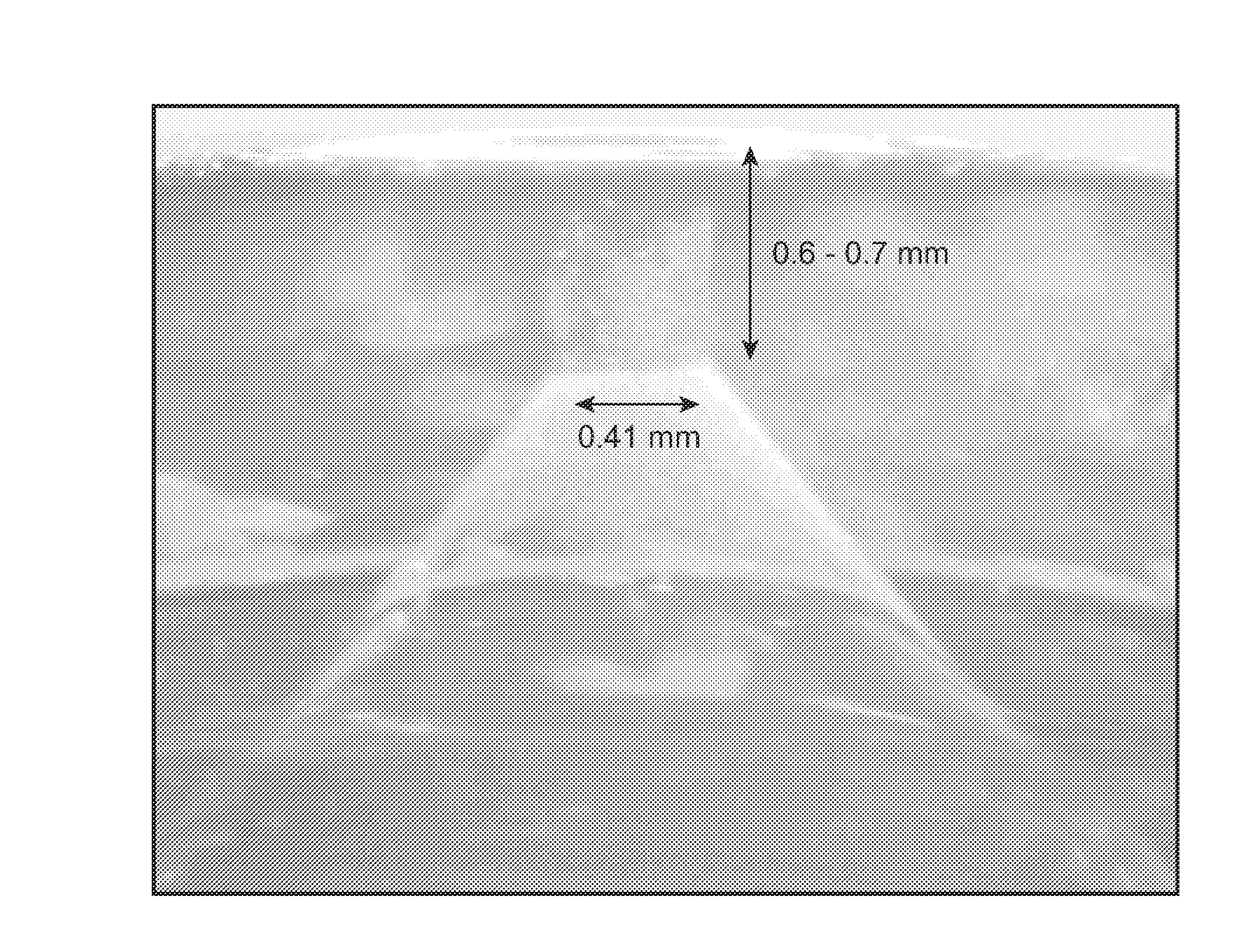
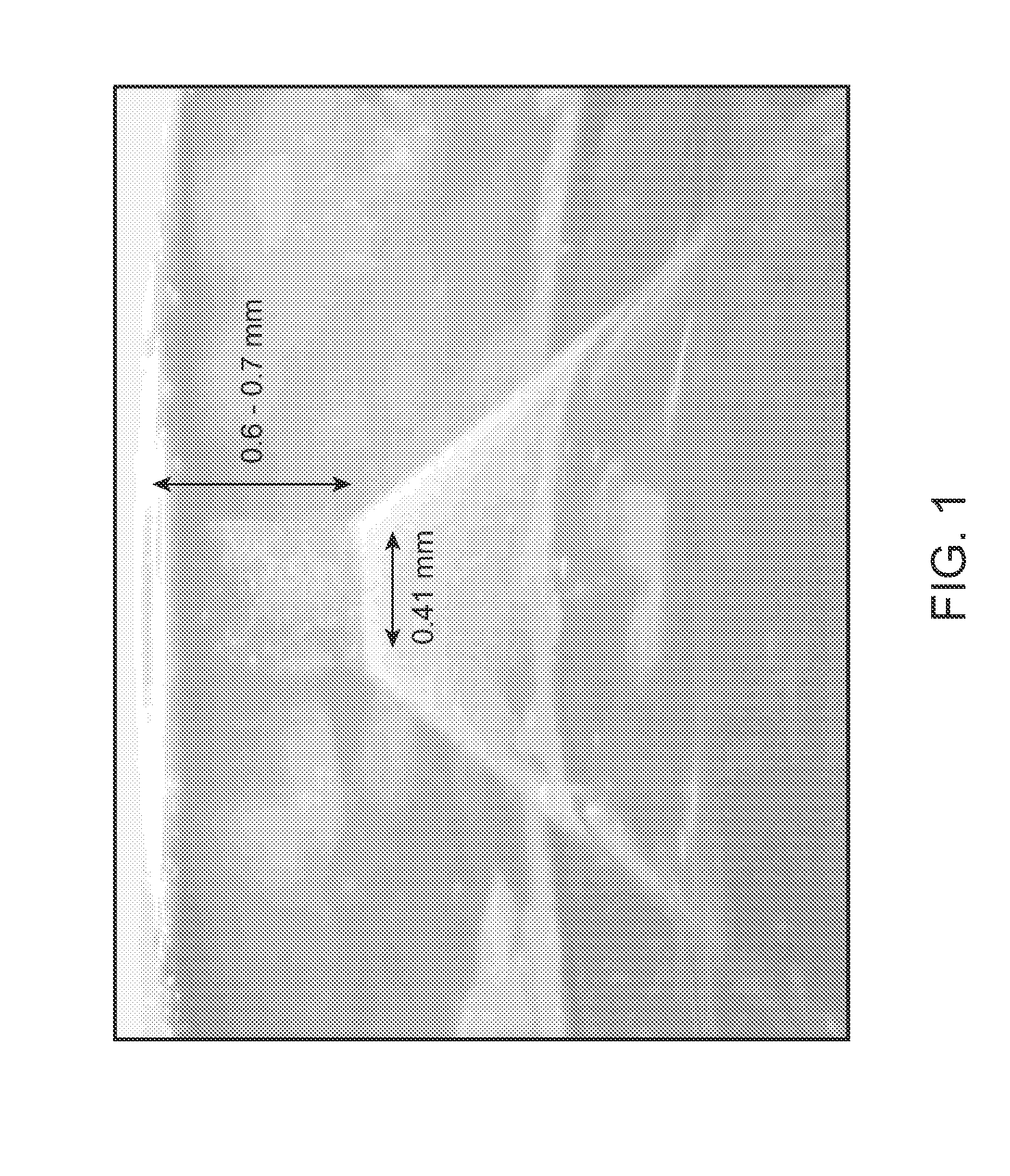
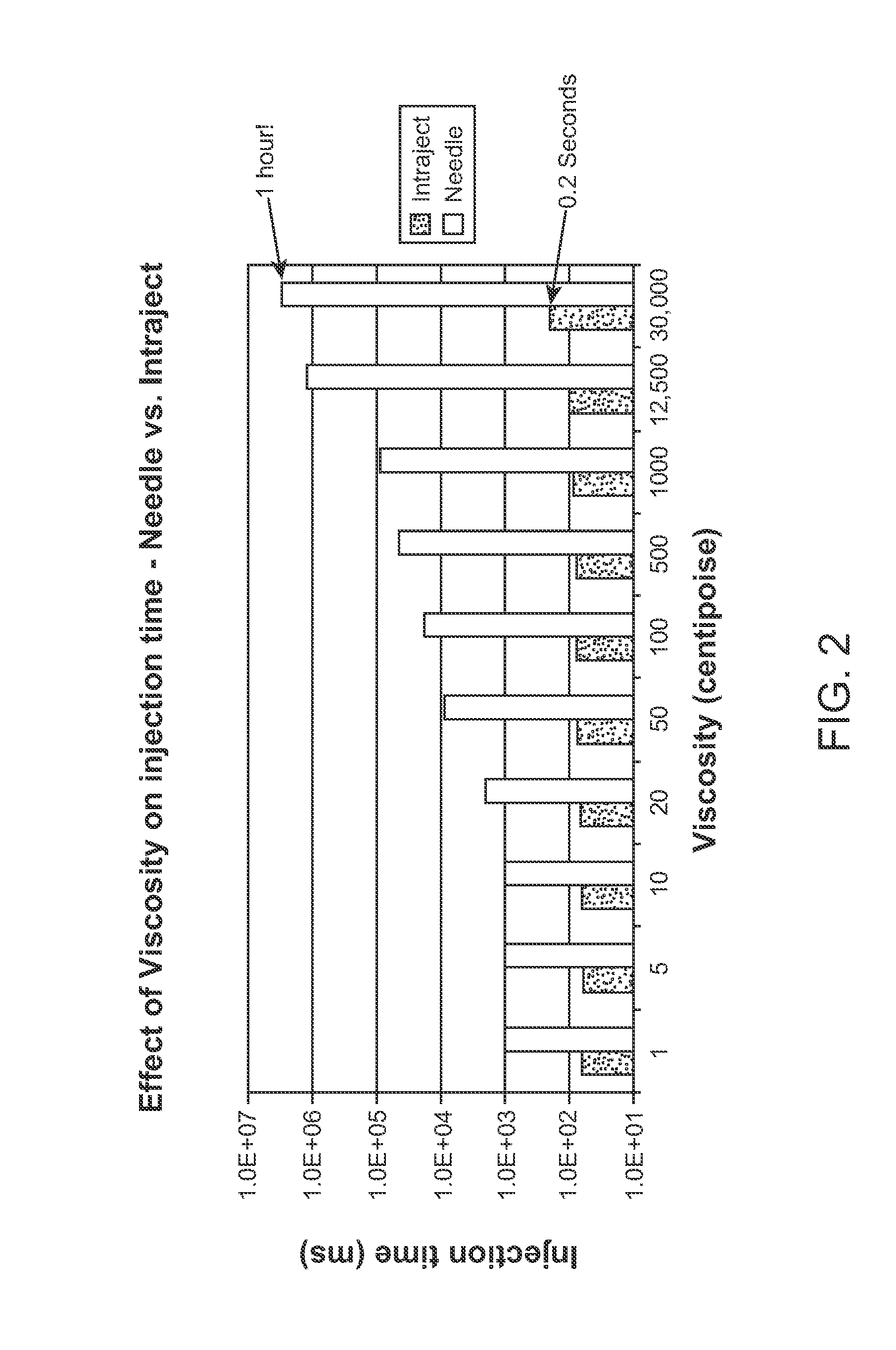
InactiveUS8066661B2Improve stabilityKeep for a long timeNervous disorderJet injection syringesNeedle freeNeedle Free Injection
Formulations are described that are viscous and will benefit from needle-free delivery at high driving pressures. Conventional delivery of these viscous formulations by hypodermic syringes is inconvenient as well as painful. Formulations include those which have a viscosity of about 5 cS or more at about 20° C. and which can have 0.5 ml or more administered by a needle-free injector in about 0.1 second±0.02 seconds.
Owner:ZOGENIX INC
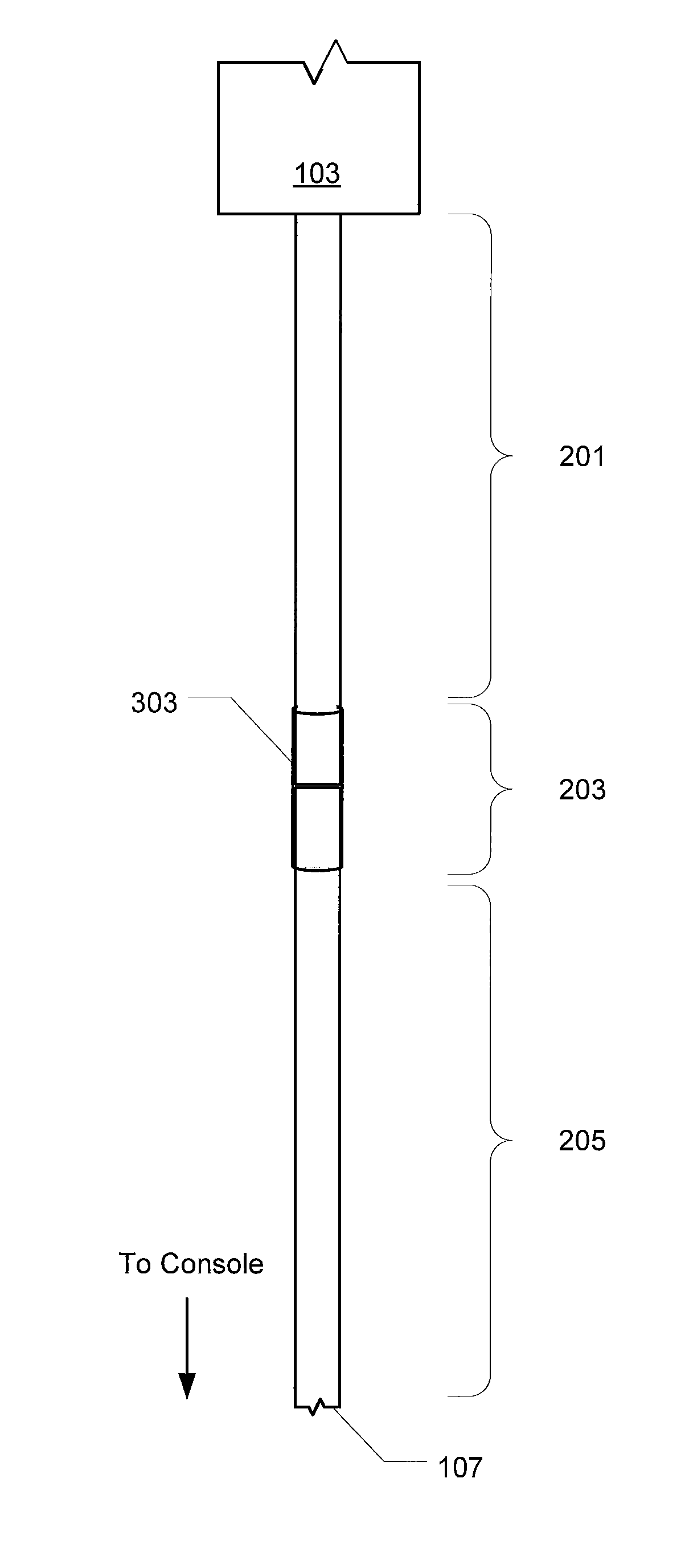
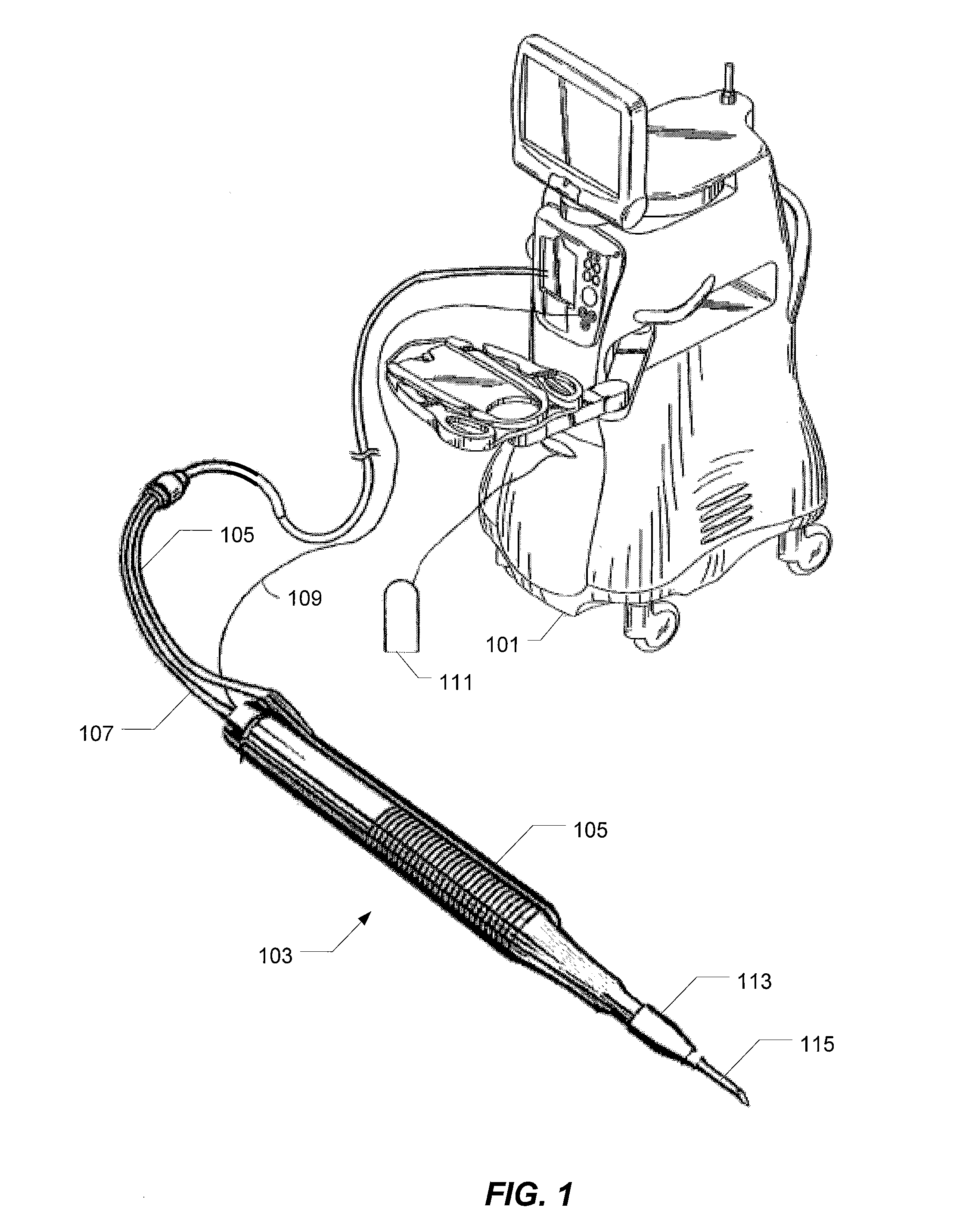
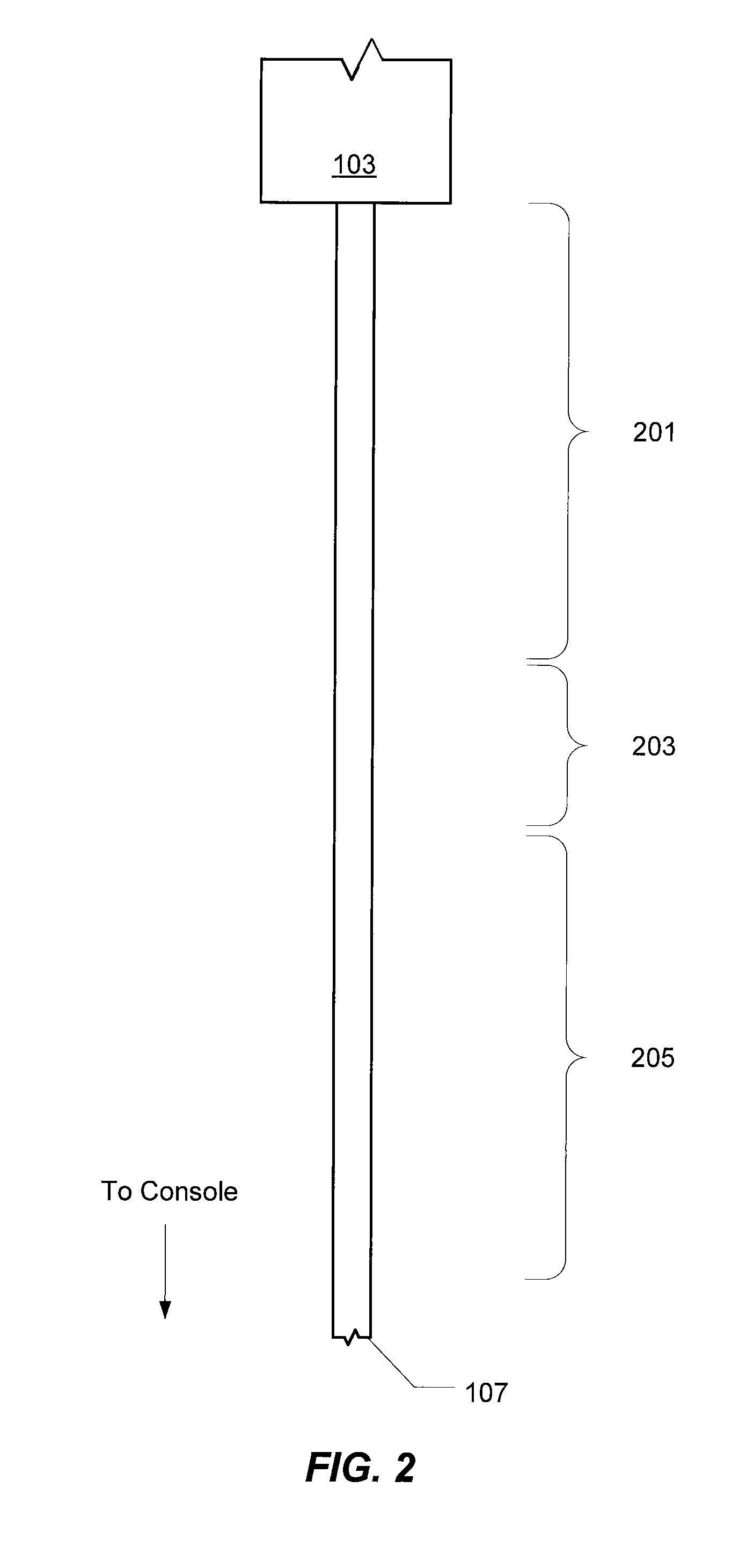
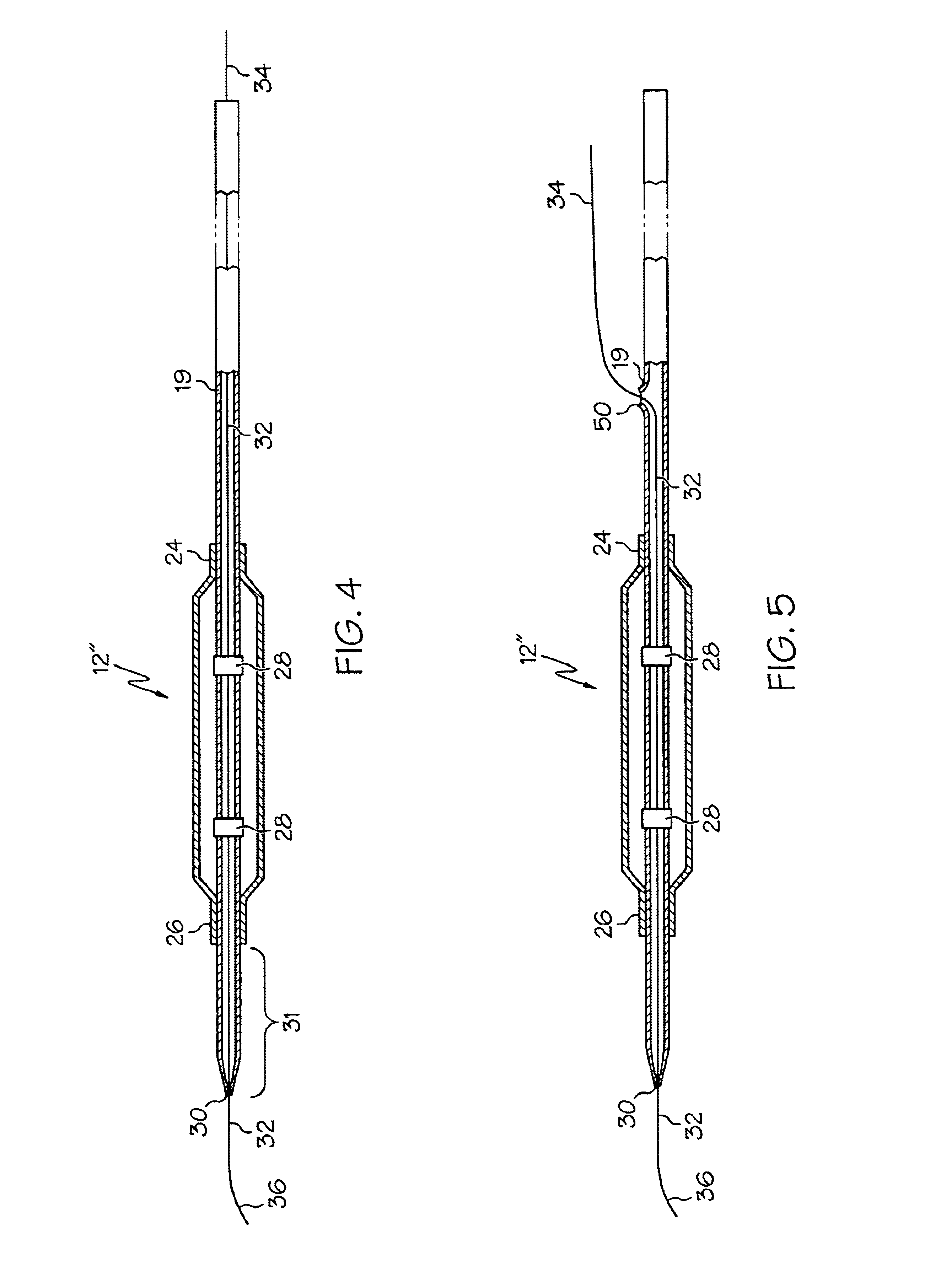
Multi-compliant tubing
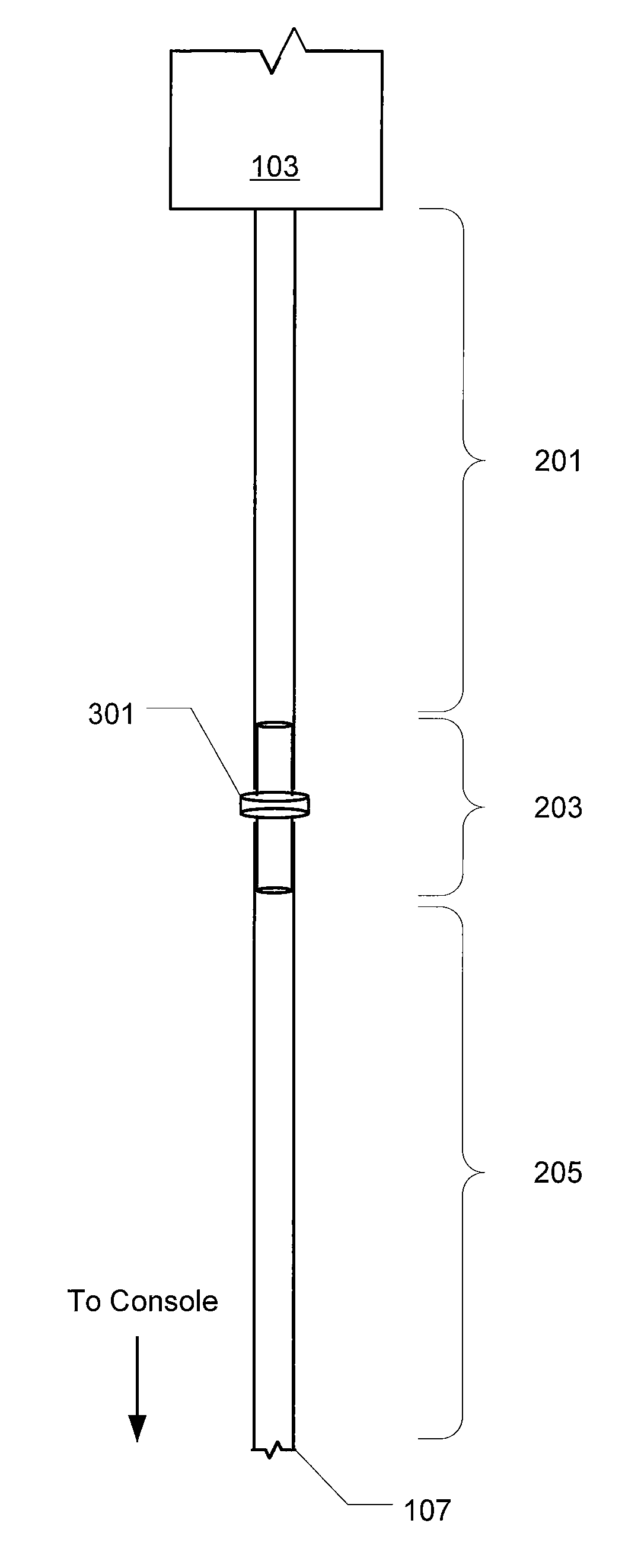
ActiveUS20100056991A1Reduce impactIncrease stiffnessEye surgeryMedical devicesMechanical engineering
In various embodiments, aspiration tubing connecting a handpiece to a surgical console may include a high compliant section, a transition section, and a low compliant section. In some embodiments, the high compliant section may have a lower durometer and / or different geometry than the low compliant section. The transition section may take a number of forms, including a connector or a continuous section of tubing that gradually increases in durometer and / or changes geometry through the length of the tubing. In the various embodiments, the high compliant section may provide flexibility near the handpiece to make the handpiece easier to hold and maneuver while the low compliant section may reduce the effects of occlusion break surge. In some embodiments, the high compliant section may include, for example, ribs, stiffening rings, a stiffening sleeve, or a stiffening sock to increase the stiffness of the high compliant section.
Owner:ALCON INC
Double layered balloons in medical devices
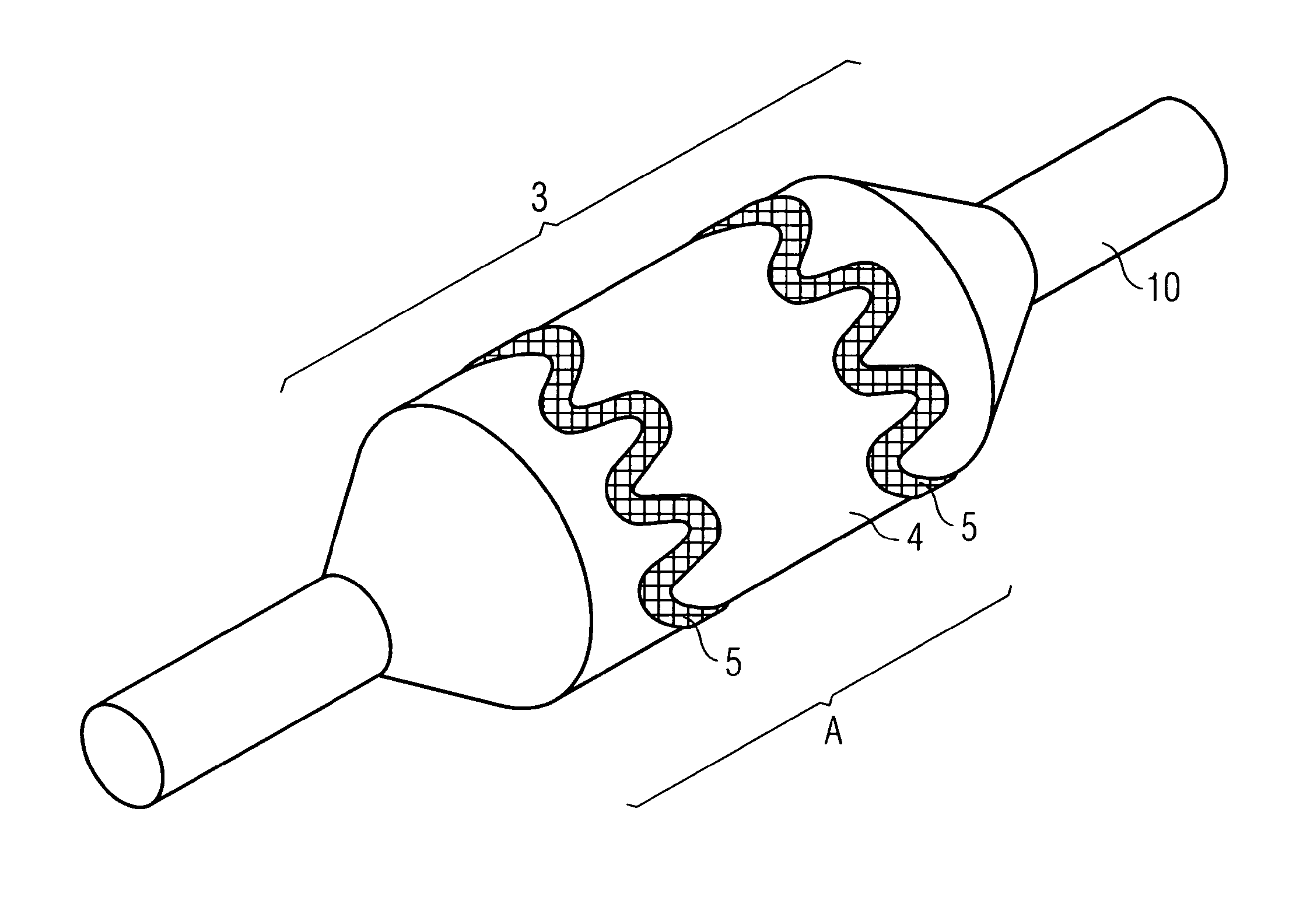
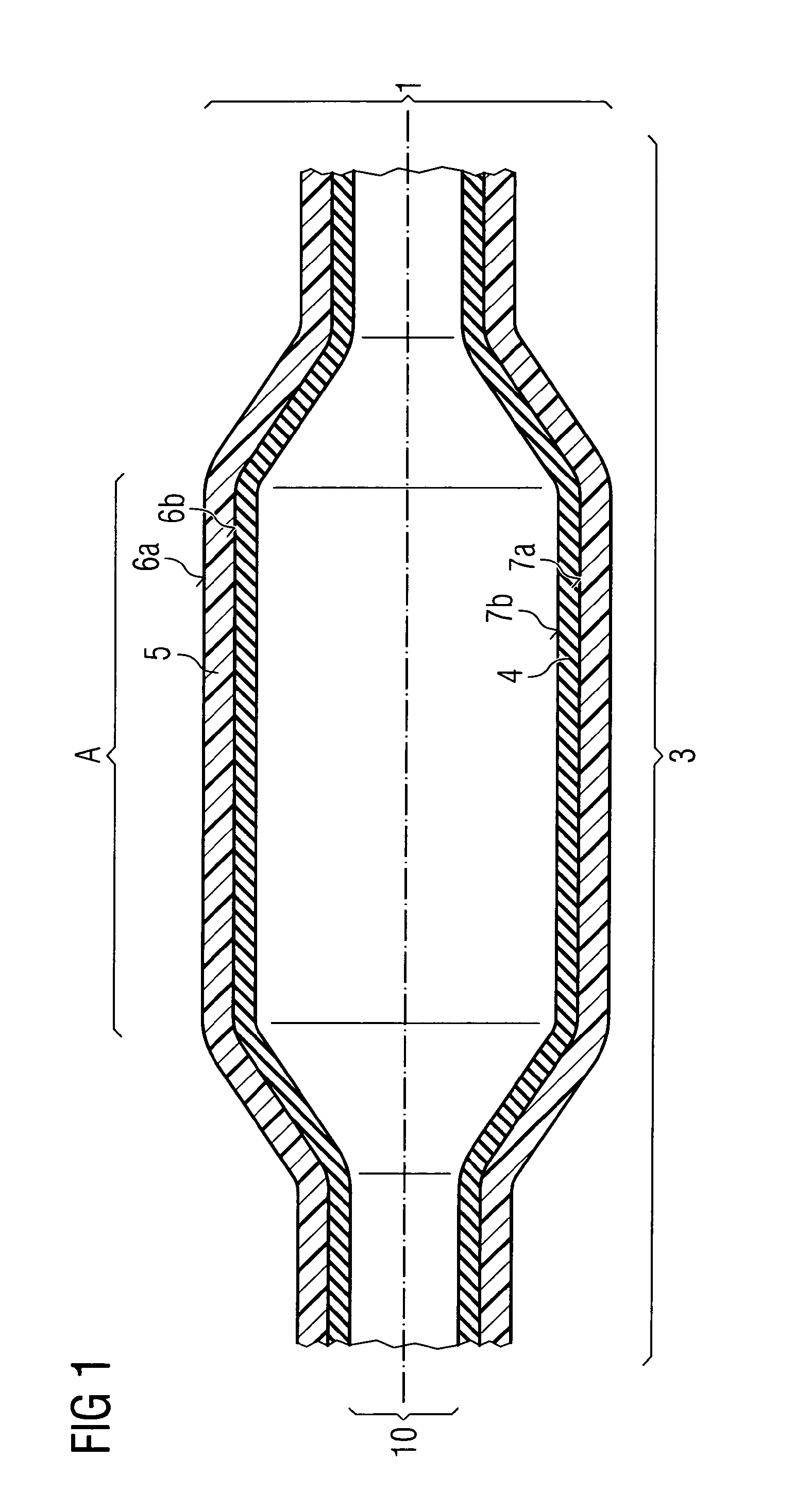
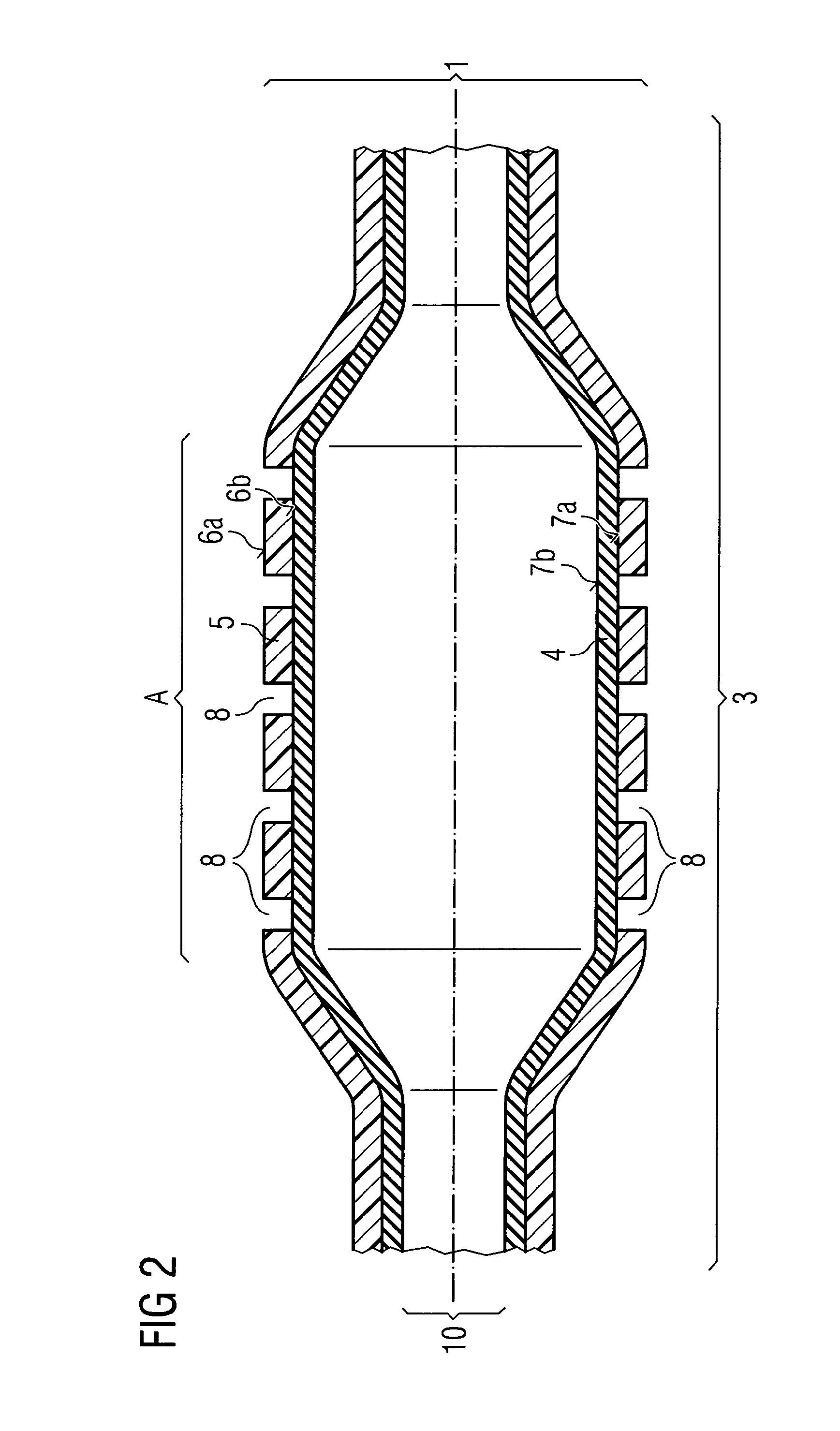
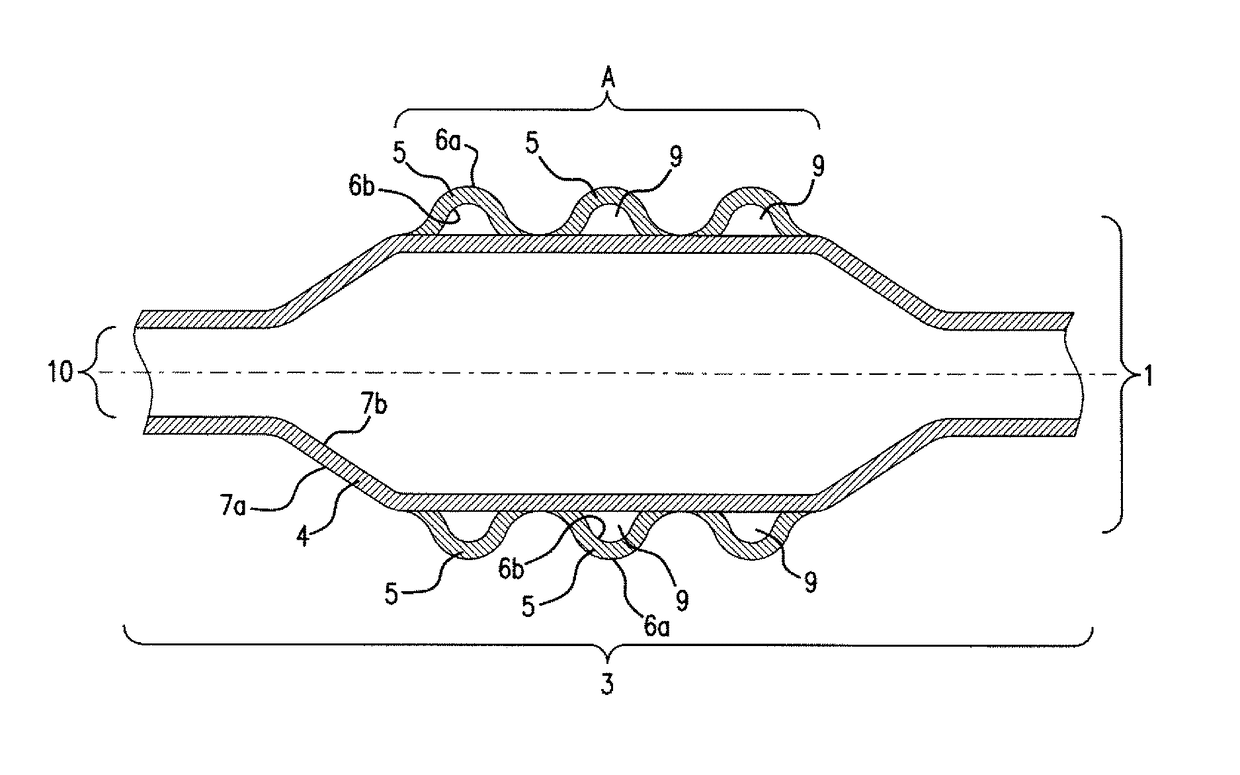
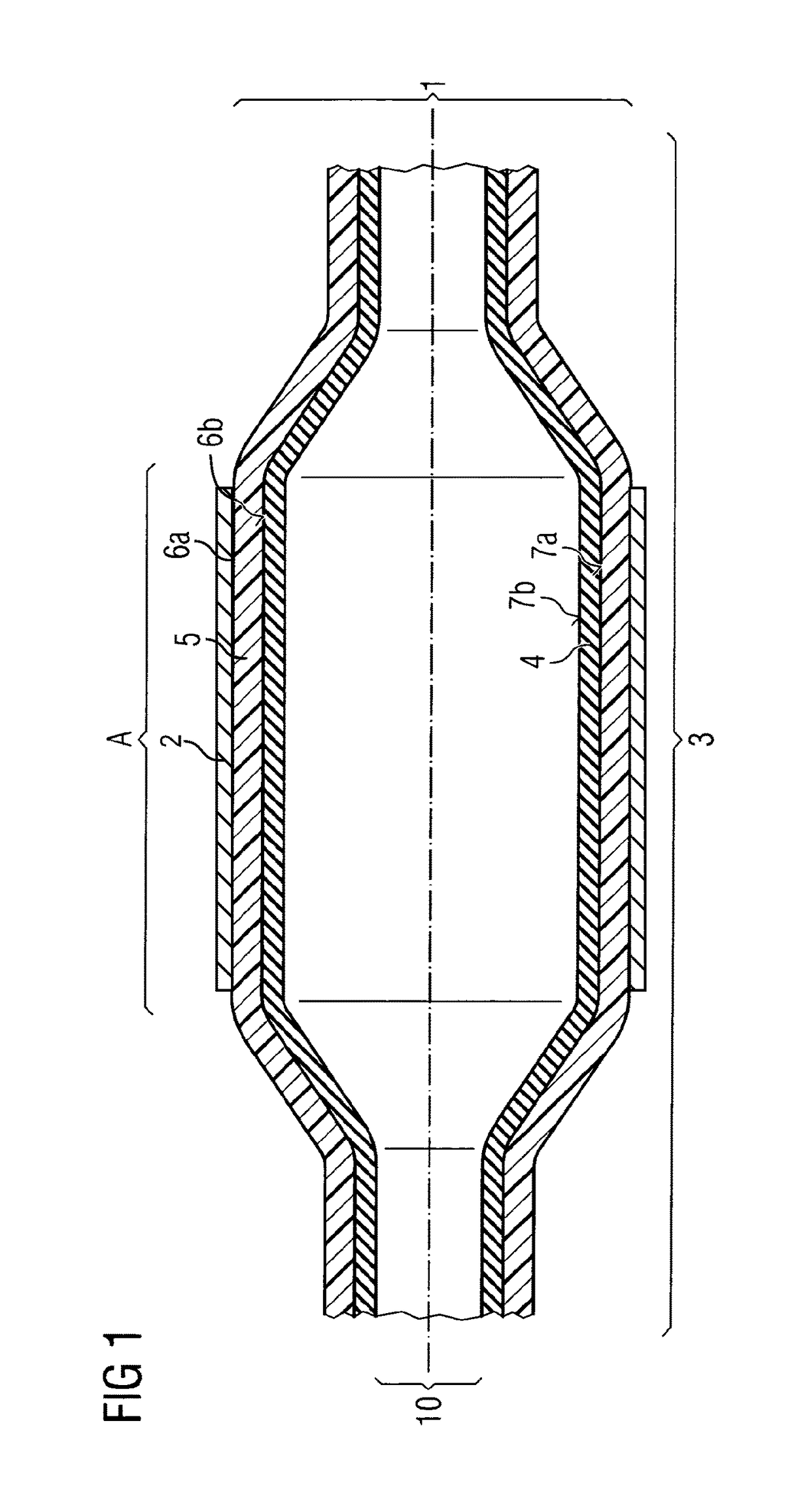
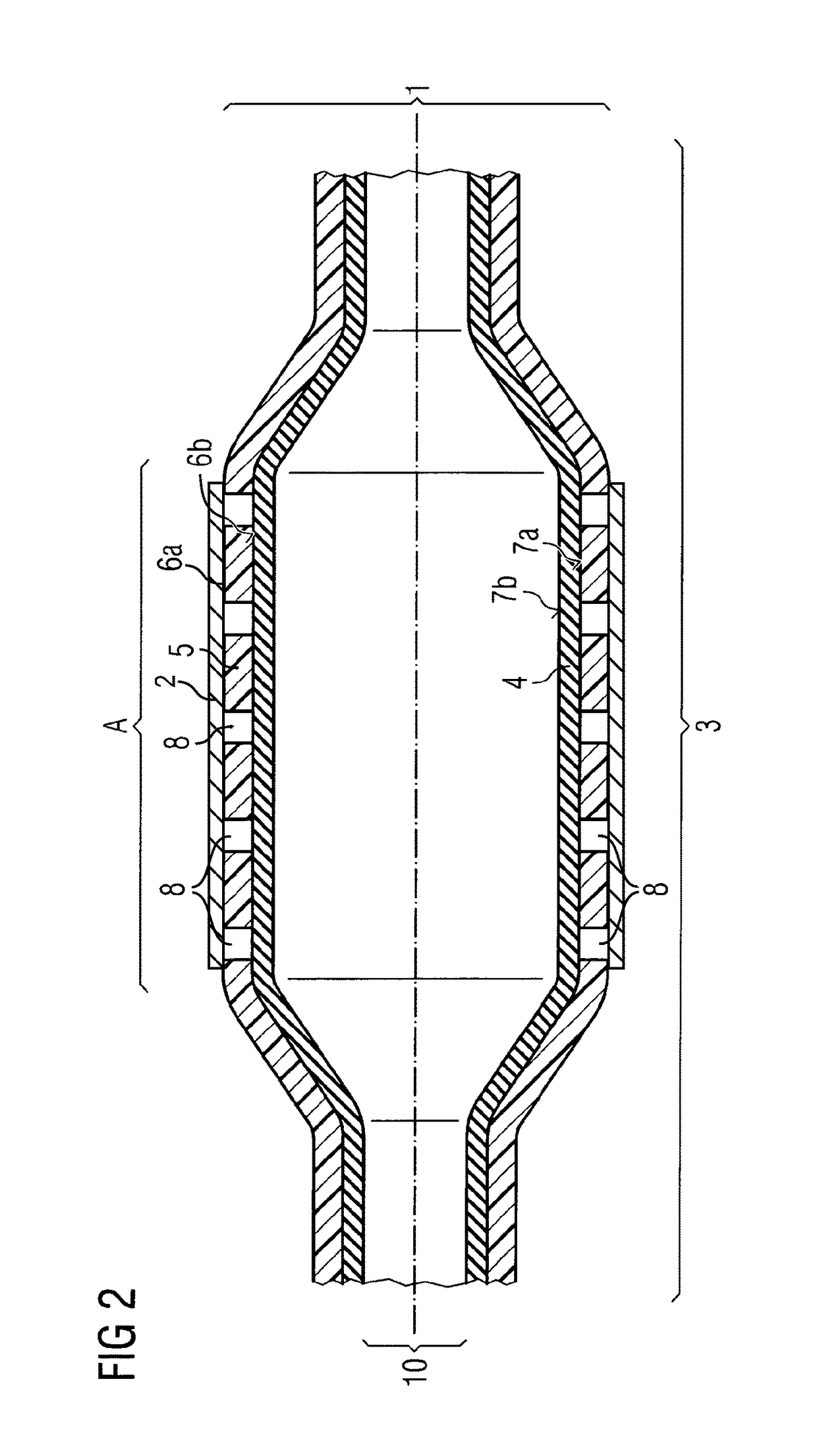
ActiveUS20110022152A1Decreases compliance rateIncrease burst pressureStentsSurgeryHuman patientBalloon catheter
The present invention refers to medical devices. Particularly it relates to stent devices and balloon catheter devices. In the most particular aspect of the invention it relates to balloon catheter devices carrying stents (2) with the medical balloon (3) comprising an inner layer (4) having a lower compliance rate and / or burst pressure than the outer layer (5) and an outer layer (5) having on the outer surface (6a) a higher adhesion strength than the inner layer (4), and its use in a variety of medical procedures to treat medical conditions in animal and human patients.
Owner:ABBOTT LAB VASCULAR ENTERPRISE
Double layered balloons in medical devices
ActiveUS9717615B2Low complianceIncrease burst pressureStentsBalloon catheterAdhesion strengthMedical treatment
Owner:ABBOTT LAB VASCULAR ENTERPRISE
Compositions and methods for localized drug delivery through mammary papillae
ActiveUS9220680B2Efficient deliveryHigh concentrationTissue culturePharmaceutical non-active ingredientsDiagnostic agentAreola
The invention provides compositions and methods for the prevention, diagnosis, or treatment of conditions affecting breast tissue. The compositions can include one or more therapeutic agents or diagnostic agents, and an effective carrier. The composition can be specifically adapted for transdermal permeation through the mammary papilla, areola, or a combination thereof, and into underlying breast tissue.
Owner:SOUTH DAKOTA STATE UNIVERSITY
Cholesterol-reducing diet
InactiveUS20080070826A1Reduce and balance cholesterol levelReduce decreaseBiocideOrganic active ingredientsLipid formationRegimen
A method of treating cholesterol imbalance, high LDLs, and other metabolic syndrome problems and symptoms of osteoarthritis. The regimen preferred embodiment includes lipids as 60% or more of daily caloric content, protein up to 10%-15% of daily caloric content; and carbohydrates up to 25% or less of daily caloric content, primarily in the form of non-starchy, low-glycemic fresh fruit and vegetables, and nuts, along with a daily intake of 35 grams of dietary fiber, with a significant percentage of this as soluble fiber; average daily cholesterol intake of less than 10 mg, and 30 grams of cocoa solids.
Owner:SELBY III HOWARD W
Balloons Having Improved Strength and Methods for Making Same
InactiveUS20080255512A1High burst resistanceSame flexibilityStentsBalloon catheterDistal portionBalloon catheter
A balloon catheter assembly includes a catheter shaft, and a balloon mounted on the catheter shaft. The balloon includes a distal neck portion, a proximal neck portion, and an inflatable body portion in between the distal and proximal neck portions. The distal and proximal neck portions are connected to the catheter shaft. The balloon also includes a fiber provided on the body portion. The fiber is oriented relative to the longitudinal axis of the body portion at an angle between about 45° and about 135°, and has a diameter of less than about 10 μm.
Owner:MEDTRONIC VASCULAR INC
Multi-compliant tubing
ActiveUS8631831B2Reduce impactIncrease stiffnessEye surgeryMedical devicesMechanical engineeringEngineering
In various embodiments, aspiration tubing connecting a handpiece to a surgical console may include a high compliant section, a transition section, and a low compliant section. In some embodiments, the high compliant section may have a lower durometer and / or different geometry than the low compliant section. The transition section may take a number of forms, including a connector or a continuous section of tubing that gradually increases in durometer and / or changes geometry through the length of the tubing. In the various embodiments, the high compliant section may provide flexibility near the handpiece to make the handpiece easier to hold and maneuver while the low compliant section may reduce the effects of occlusion break surge. In some embodiments, the high compliant section may include, for example, ribs, stiffening rings, a stiffening sleeve, or a stiffening sock to increase the stiffness of the high compliant section.
Owner:ALCON INC
Varying Material Properties of a Single Fluidic Line in Ophthalmology Tubing
Twin bore ophthalmologic tubing includes first and second tubes. The second tube has portions of differing hardness with one of the portions being at an end of that tube. The second tube has a portion at its other end with about the same hardness. Portions of the first and the second tubes may have about the same hardness. In some embodiments, portions of the first and second tubes with the same hardness can correspond to each other along the tubing. One of the portions can have a hardness of 80 to 90 shore A while the other portion can be 60 to 70 shore A. In some embodiments, portions with 60 to 70 shore A hardness can be about six to twelve inches long.
Owner:ALCON INC
Blended polyurethane interventional balloon
InactiveUS6951674B1High and mean burst pressure characteristicLow complianceSynthetic resin layered productsThin material handlingGlass transitionHeat treated
A combination of urethane polymeric components provides desired characteristics in forming medical instruments such as catheters and balloons for dilatation catheters. For example, a balloon material is formed form a blend of urethane polymeric components, including a first polymeric component having a first glass transition temperature and a second polymeric component having a second glass transition temperature. The first polymeric component can be branched or straight chain of thermoplastic polyurethane compound having a glass transition temperature polyurethane compound having a glass transition temperature higher than normal human body temperature. The second polymeric component can be a second thermoplastic polyurethane having a glass transition temperature equal to or lower than normal human body temperature. The polymeric blend can be heat treated to further enhance the properties or stability of the balloon material, including significantly increasing burst pressure.
Owner:BOSTON SCI SCIMED INC
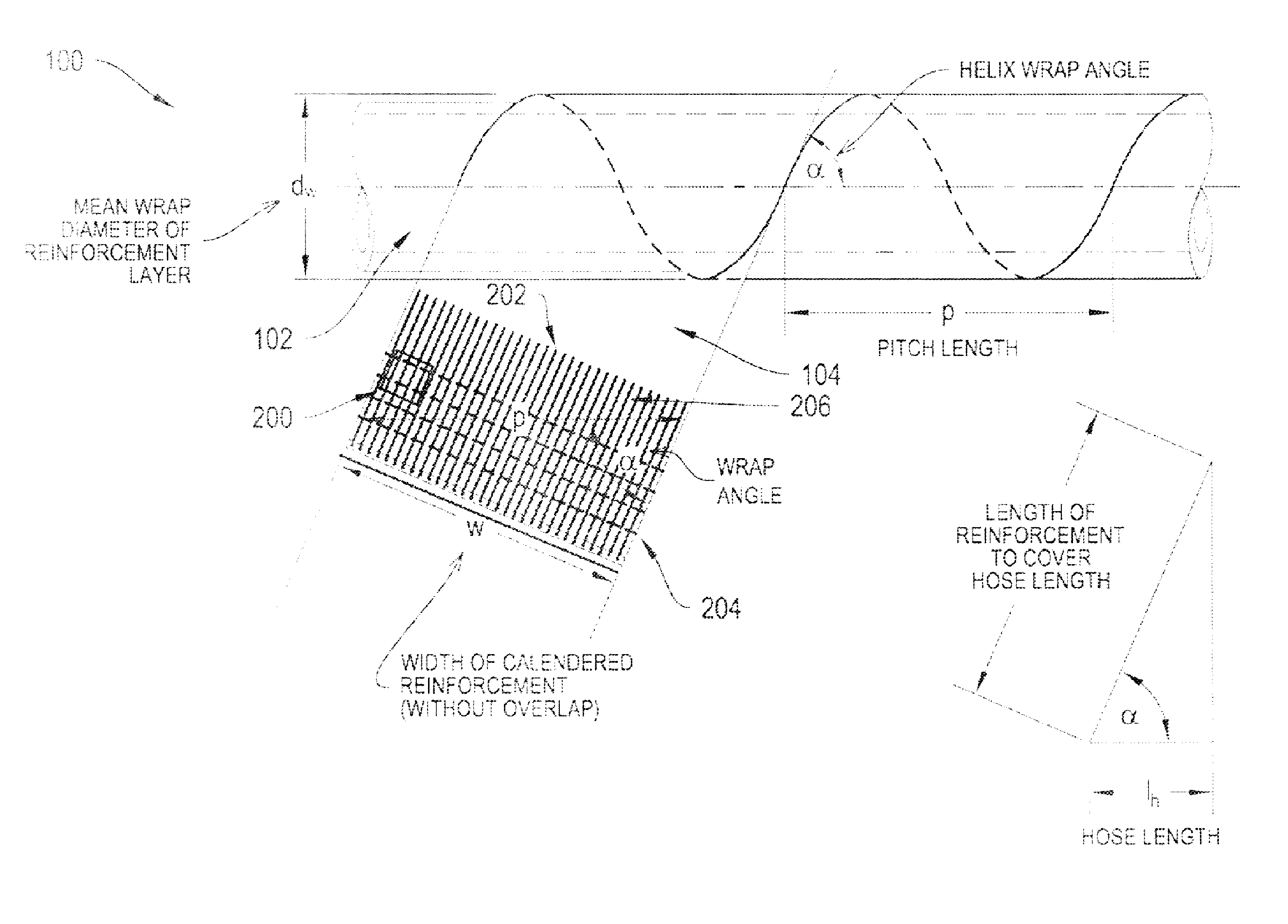
Multi-Layered Stretchable Hose
ActiveUS20160369919A1Facilitate high-throughputIncrease the number ofCorrosion preventionSeismic signal receiversElectrical conductorEngineering
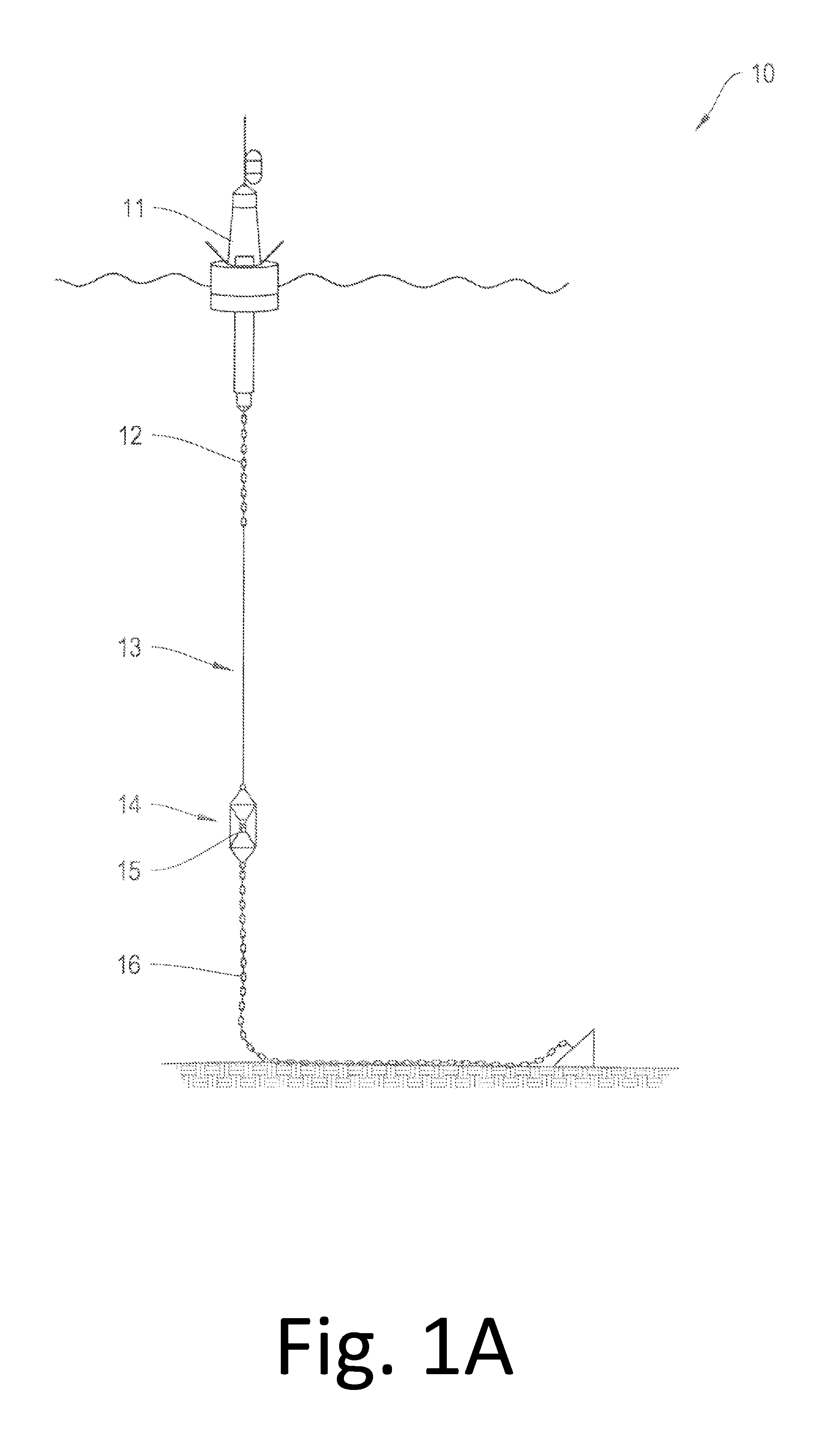
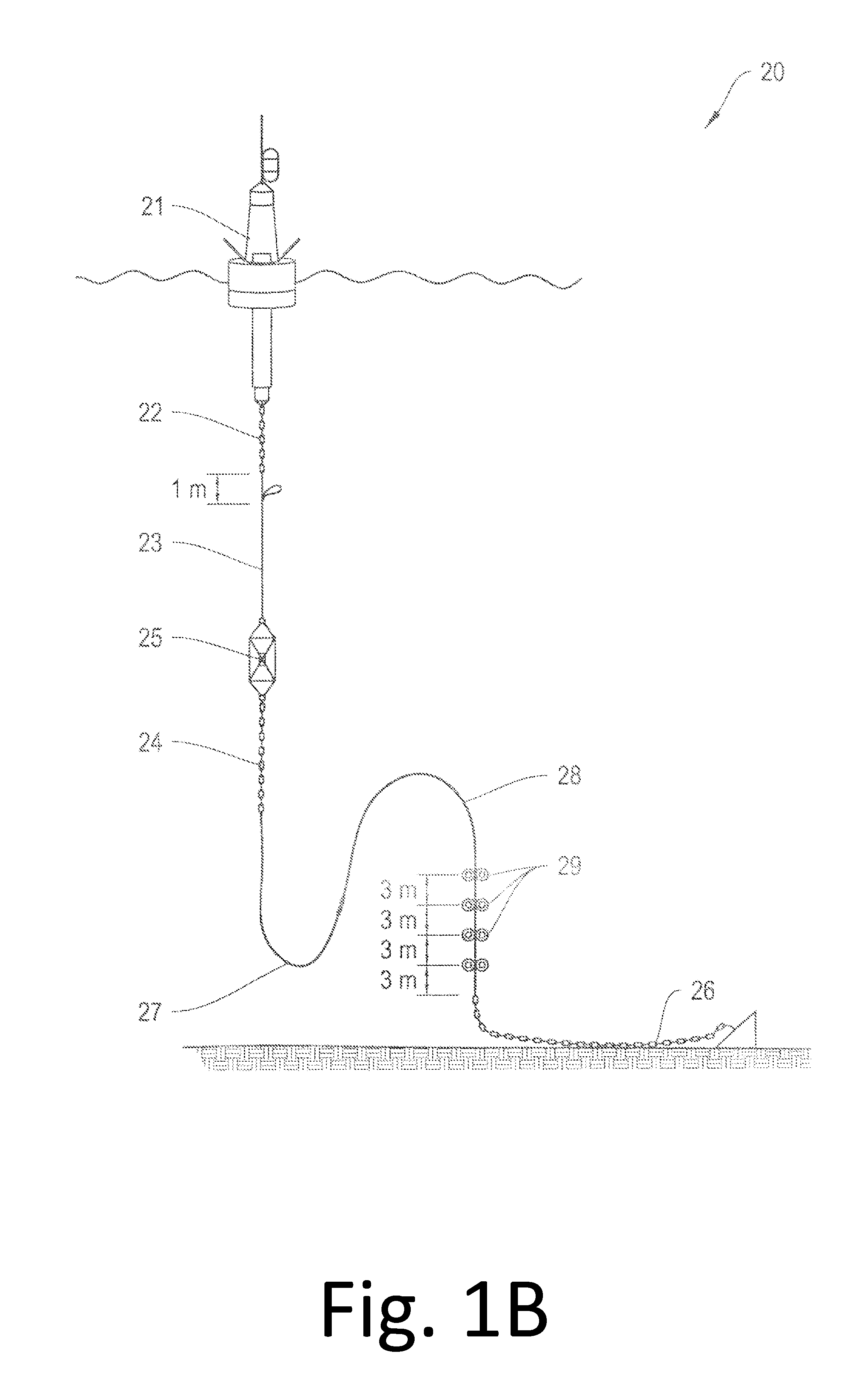
The systems and methods described herein relate to an improved stretchable hose apparatus which enables the transmission of high-throughput signals along several conductors within a conductor cable. In particular, the stretchable hose enables consistent high speed communications and high power transmission between a buoyant object and an optional underwater device in all weather conditions by permitting compression of the conductor cable in a first range of hose extensions and limiting elastic elongation of the conductor cable in a second greater range up to full hose extension.
Owner:WOODS HOLE OCEANOGRAPHIC INSTITUTION
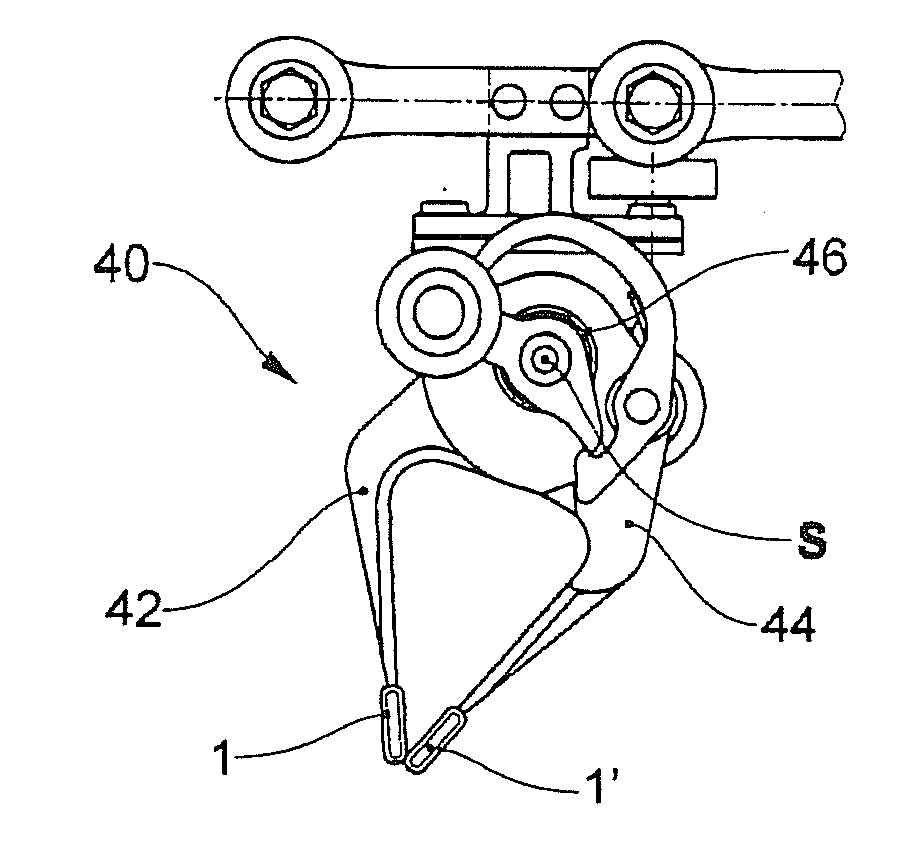
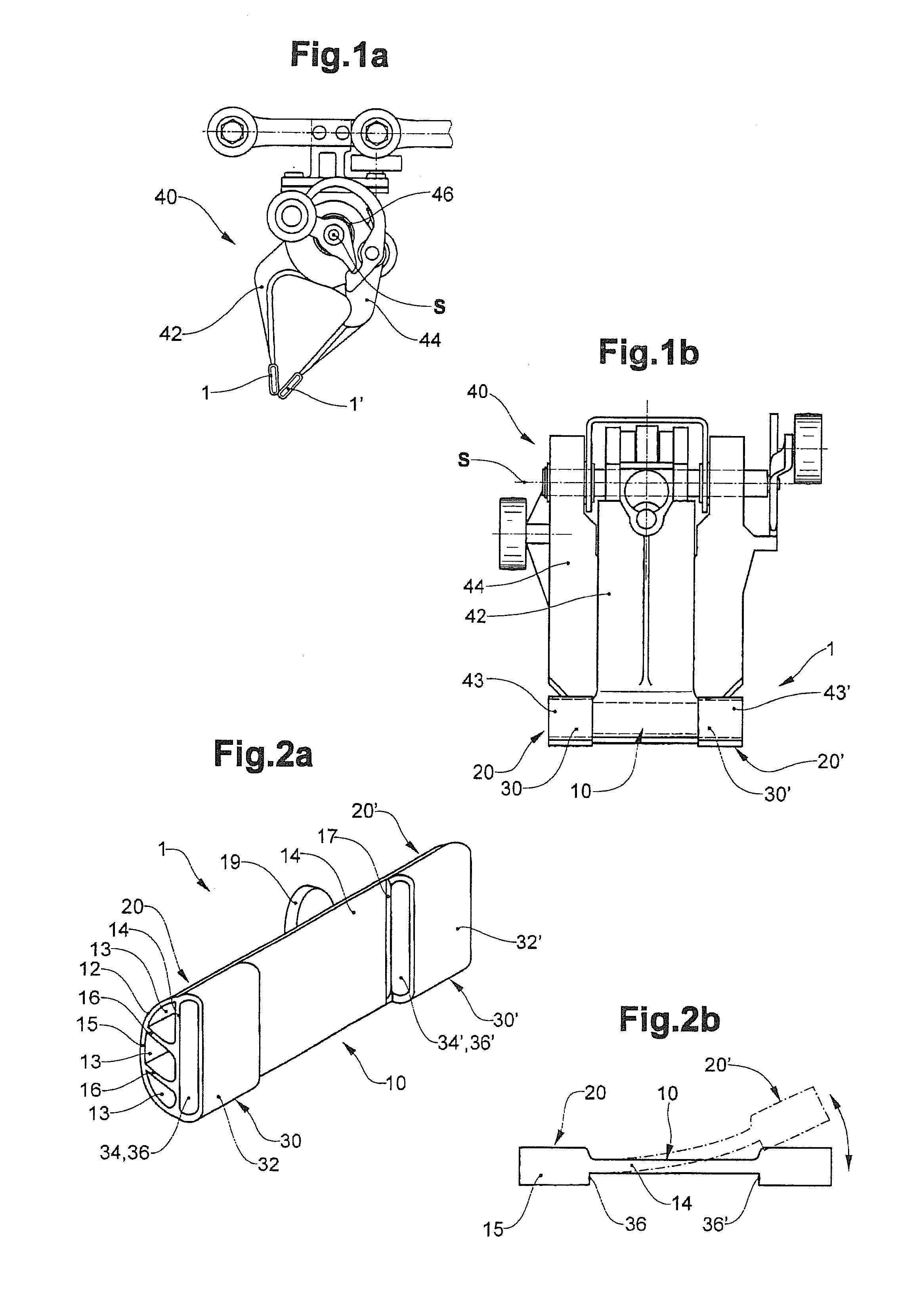
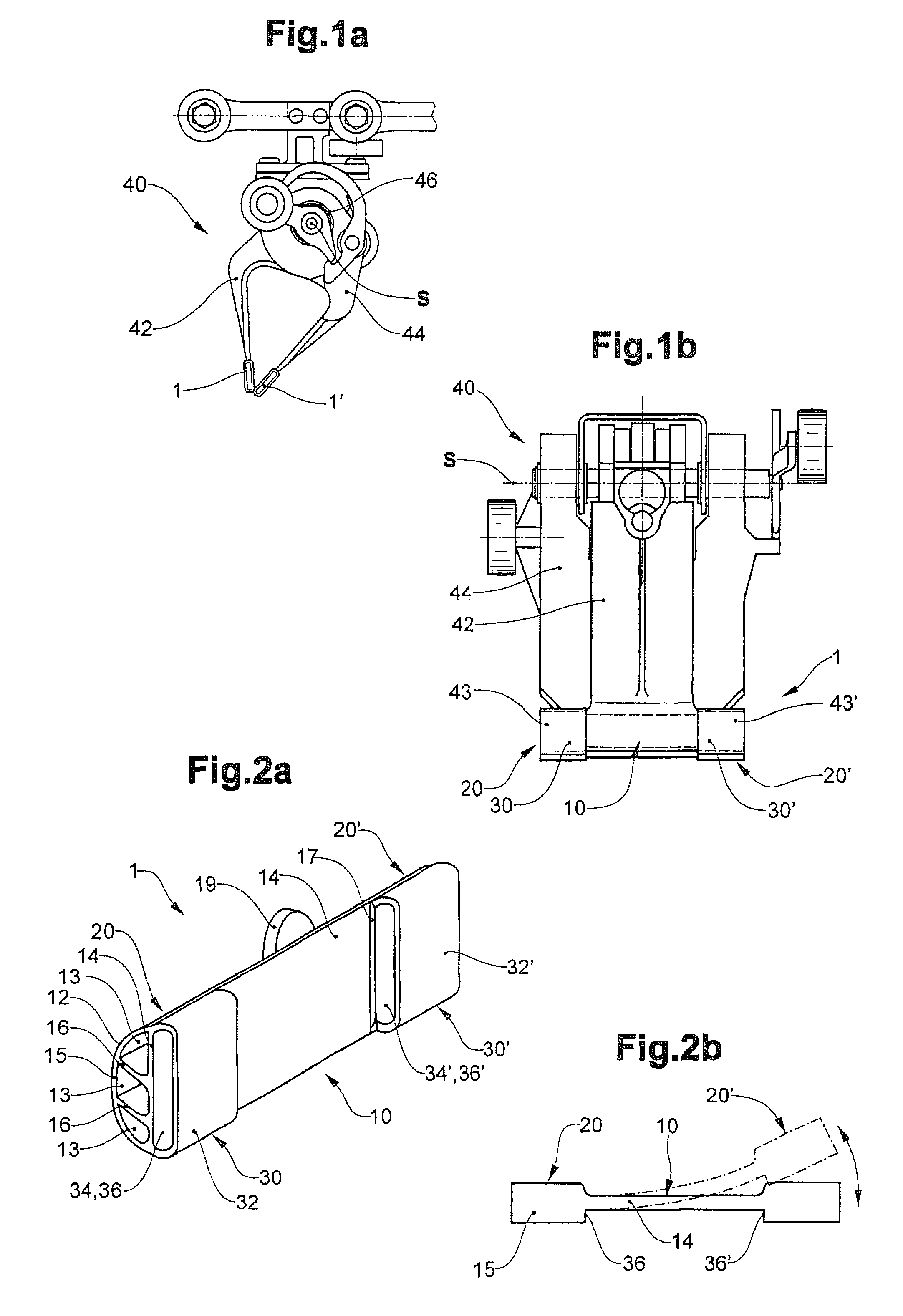
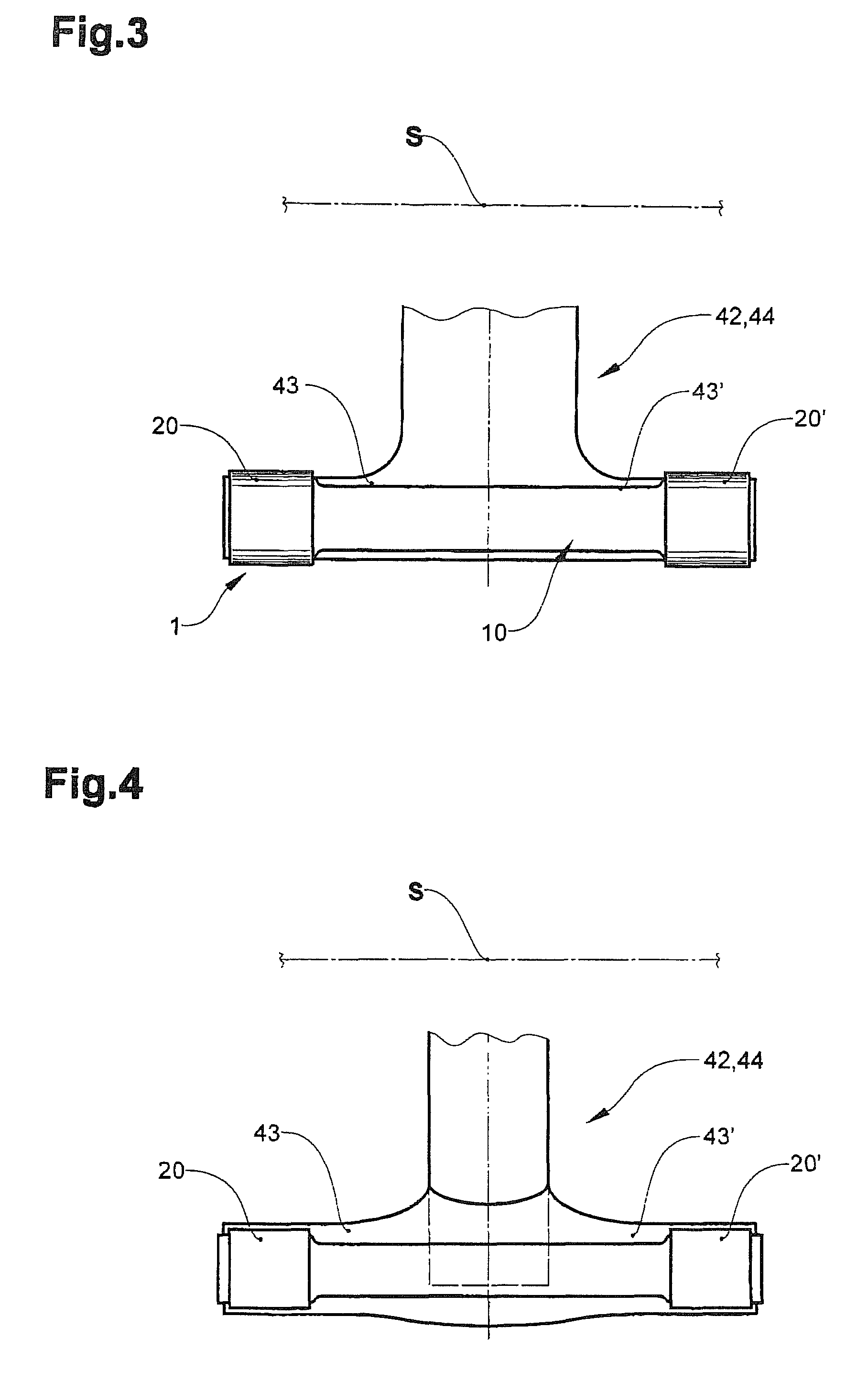
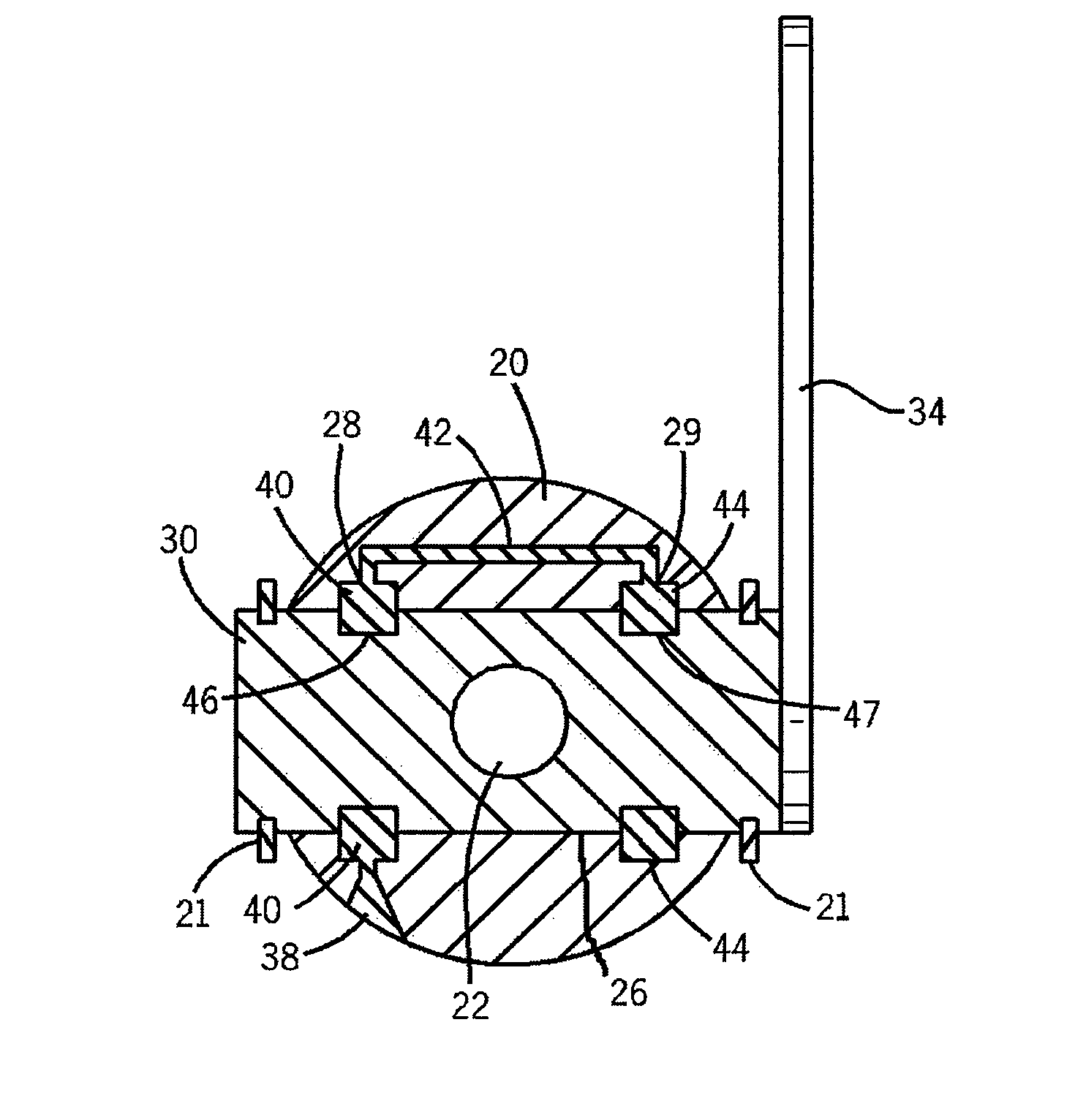
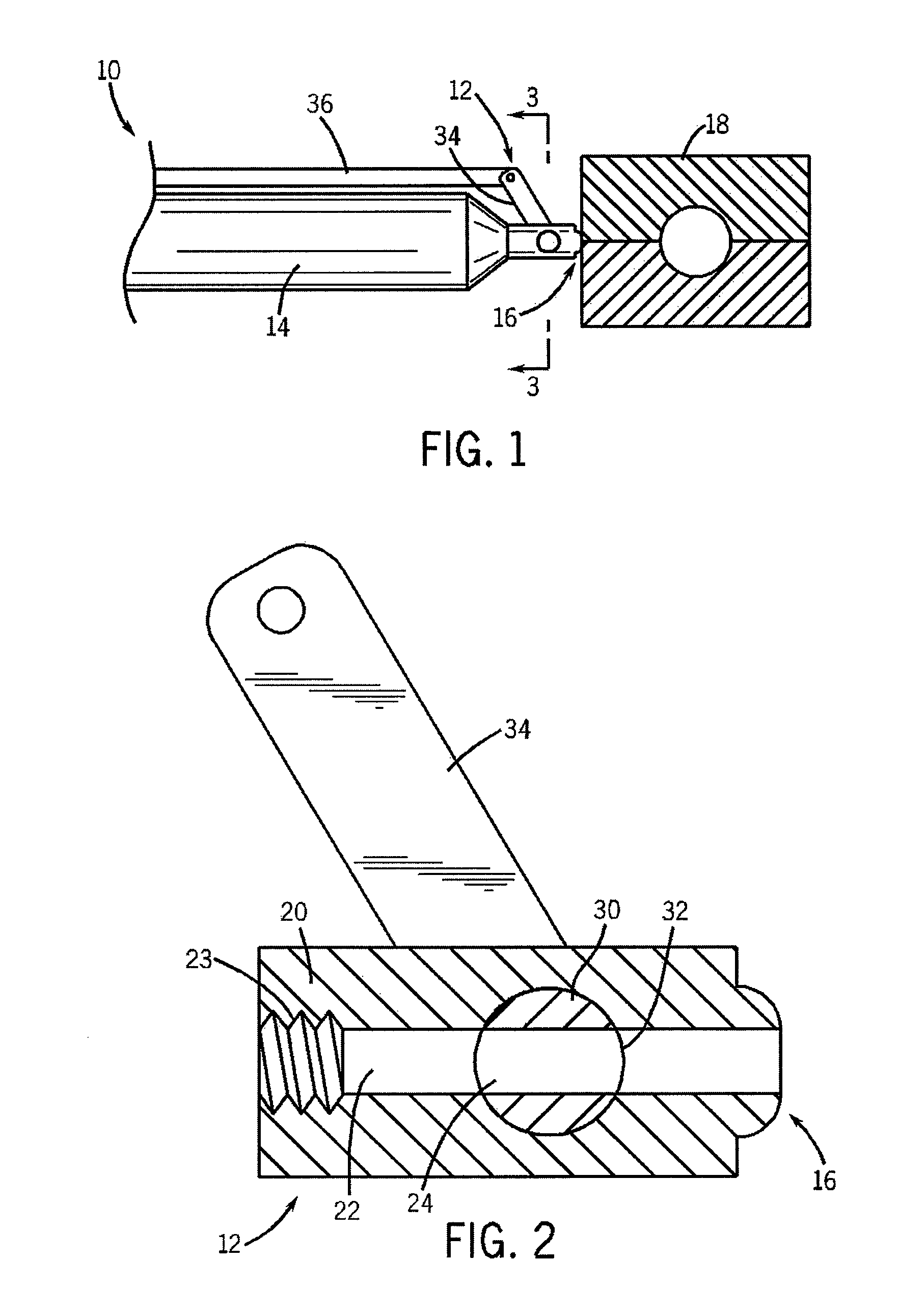
Clamping element for fitting on a clamping tongue of a gripper as well as gripper fitted with such a clamping element
Owner:FERAG AG
Catheter balloon
An expandable medical device or component thereof including a tubular body formed of a wrapped sheet of porous polymeric material fused together, the tubular body having a fused seam at an angle relative to the longitudinal axis of the tubular body which changes along the length of the tubular body from a first angle to a second angle greater than the first angle. The sheet of porous polymeric material is wound and then fused together such that the winding angle is less in a first longitudinal section of the tubular body compared with the winding angle in a second longitudinal section of the tubular body, in order to provide the second section with greater resistance to expansion (i.e., lower compliance) than the first section.
Owner:ABBOTT CARDIOVASCULAR
Low compliant catheter tubing
ActiveUS20100130927A1High complianceStrength and modulus be increaseStentsBalloon catheterPolytetramethylene glycolPolyamide
combination of at least two polyamides. The catheter component can be made from a blend of the two polyamides, or a co-extrusion of the two polyamides with an inner layer and an outer layer. The first polyamide has a Shore D durometer hardness of more than seventy seven (77), and can be selected from various transparent amorphous nylons having an amorphous segment such as an aliphatic segment, an aromatic segment, or a cycloaliphatic segment. The second polyamide has a lower durometer hardness than the first polyamide, and preferably less than 73, and can be a block copolymer of nylon and polytetramethylene glycol. Both polyamides have the same amide block or segment, e.g. nylon 12. Adding small amounts of the high hardness polyamide can reinforce the low polyamide at the amorphous segments, thereby increasing the overall strength of the material.
Owner:ABBOTT CARDIOVASCULAR
Clamping element for fitting on a clamping tongue of a gripper as well as gripper fitted with such a clamping element
InactiveUS8220853B2Low complianceReliable holdGripping headsLoad-engaging elementsEngineeringMechanical engineering
Owner:FERAG AG
Nozzle shutoff for an injection molding machine
InactiveUS8286940B2Improved valvePrevent stringPlug valvesPipe elementsInjection molding machineThermoplastic materials
The present invention is directed to an injection molding valve configured to prevent the leaking or stringing of injected thermoplastic from the valve. The valve includes a housing having a first bore along a length thereof and a second bore intersecting the first bore. A rotatable member is inserted through the second bore and is in communication with a lever for the actuation thereof. The rotatable member includes a hole therethrough such that when the hole is aligned with the first bore, thermoplastic may travel freely through the first bore. The second bore further includes at least one groove in communication with a port for receiving injected thermoplastic. The injected thermoplastic is hardened to form an seal therein and configured to prevent thermoplastic from leaking out of the valve housing.
Owner:MGS MFG GROUP
Orally taken granule for treating malaria and preparation method thereof
InactiveCN106309406ALow complianceSweet tasteOrganic active ingredientsPharmaceutical non-active ingredientsDocument preparationSucrose
The invention discloses an orally taken granule for treating malaria and a preparation method thereof. The orally taken granule contains artesunate, coating material, stabilizer, filler, preservative and corrigent. 1000mg of oral liquid contains 60g of artesunate, 5g of coating material, 0.35g of stabilizer, 550g of filler, 1.2g of preservative and 5g of corrigent. The orally taken granule can overcome the shortage of infant medicines and can meet the requirement of infant for taste; plenty of documents and practices prove that the medicinal saccharose is taken as main filler, and meanwhile, the covering function of different coating materials for the bitter and the guiding functions of acesulfame and natural plant essence for gustation are surveyed, and the optimal tasty formula is screened through plenty of blinding data model researches.
Owner:FRONT PHARM PLC
I-channel surface-mount connector
InactiveUS6860003B2Lower impedanceQuality improvementPrinted circuit assemblingLine/current collector detailsSurface mountingInterconnection
In accordance with the invention, a low impedance surface-mount connector comprises a length of cylindrical rod having an I-shaped cross section. The device permits interconnection by pick-and-place techniques, and the interconnection has advantageous qualities of low resistance, low inductance, mechanical compliance and ease of manufacture. A first circuit device having one or more circuit components is interconnected with a second circuit device by surface mounting such connectors on the first circuit device, providing corresponding solder pads on the second circuit device, and mounting the connectors of the first circuit device onto the pads of the second.
Owner:POWER ONE INC +1
Viscous formulations and their use in needle-free injection
InactiveUS20110196336A1Improve stabilityKeep for a long timeNervous disorderJet injection syringesNeedle freeNeedle Free Injection
Formulations are described that are viscous and will benefit from needle-free delivery at high driving pressures. Conventional delivery of these viscous formulations by hypodermic syringes is inconvenient as well as painful. Formulations include those which have a viscosity of about 5 cS or more at about 20° C. and which can have 0.5 ml or more administered by a needle-free injector in about 0.1 second±0.02 seconds.
Owner:ZOGENIX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com