Pharmaceutical compositions from carapa guianensis
A composition and medicine technology, applied in the field of tetranortriterpenoids, can solve problems such as no analgesic activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Extract preparation
[0090] (a) Oil of Neem Guyana
[0091] The oil of Carapa guianensis used in the present invention is obtained by mechanical pressing of the seeds. When used in experiments, heat aliquots of oil at 40 °C until they are completely melted and dilute in sterile saline solution and Tween 20 at a ratio of 1 μL Tween / mg total mass. When preparing therapeutic solutions, the oil needs to be heated. To ensure the chemical stability of the product, each aliquot of oil was heated at 40°C at least twice.
[0092] (b) Tetranortriterpenoids
[0093] The tetranortriterpenoids of the present invention can be obtained from the oil of the oleifera oleifera or the residue of the seeds of the oleracea oleifera. The methods used in each case are conventional methods.
[0094] The oil of Oleifera oleifera was extracted with acetonitrile in three steps—minimum, stirred and decanted, and the supernatant collected. The supernatant was filtered and evaporated in a rota...
Embodiment 2
[0099] Preparation of Solutions, Drugs and Formulations
[0100] Solutions, drugs and formulations used in this experiment were prepared as described below.
[0101] (a) Preparation of drug
[0102] Promethazine chloride was soaked in tablets (Aventis), weighed and dissolved in a sterile solution of 0.9% NaCl prepared immediately before use. Cyproheptadine (Sigma) was dissolved in water. Sulpyrine was diluted and diclofenac was dissolved in fresh water (0.22 μm). Dexamethasone (Sigma), WEB 2170 (Boehringer-Ingelheim) and HOE 140 (Sigma) were dissolved in sterile NaCl (0.9%) solution. Promethazine cream (Rhodia Farma) was applied directly to the animal's paw.
[0103] All drugs are prepared immediately before use.
[0104] (b) Preparation of solution
[0105] brine
[0106] NaCl 0.9g
[0107] Distilled water (appropriate amount to) 100.00mL
[0108] Adjust pH to 7.2-7.4
[0109] heparinized saline
[0110] Saline 100.00mL
[0111] Heparin 2,000UI
[0112] Adjust pH...
Embodiment 3
[0165] In vivo assay of antiallergic activity of orally administered oil of Carapa guianensis and tetranortriterpenoids.
[0166] For all in vivo methods described below, Swiss male mice, weighing 18 to 25 g, and / or male Wistar rats, weighing 200 to 300 g, were used. The animals were funded by the Central Biotery of Fundac o Provided by Oswaldo Cruz and kept in the biotery of the Laboratório de Farmacologia Aplicada, Far-Manguinhos until use. Animals had free access to water and animal chow and were placed at 25°C with an alternating cycle of light and dark for 12 hours. The animals were treated with repellant (Mebendazole, 20 mg / 1000 mL water) for 3 days and used only for experiments after 3 days interval. All experimental methods were performed in accordance with the Ethics of Animal Experimentation by Fundagao Oswaldo Cruz, RJ.
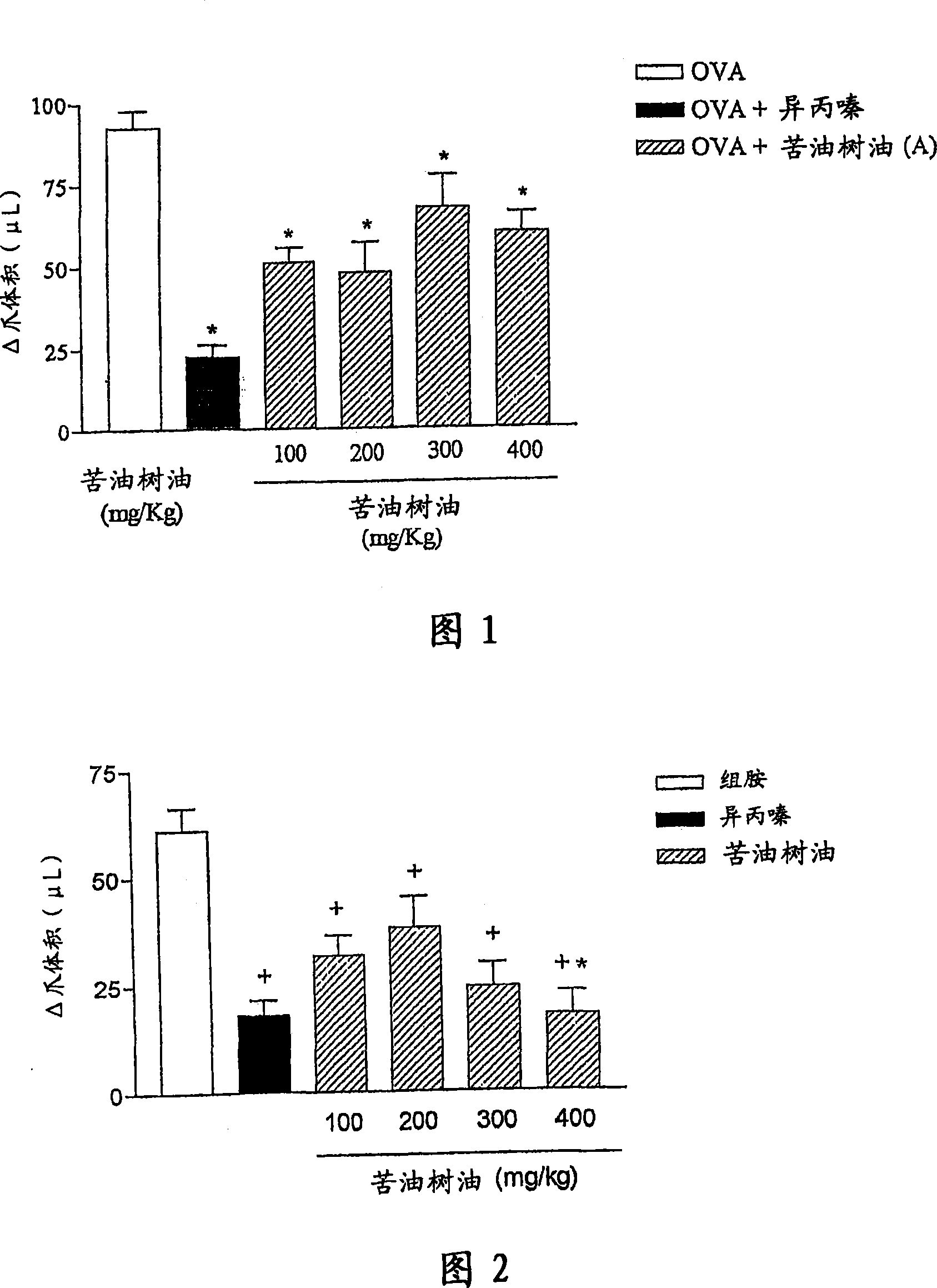
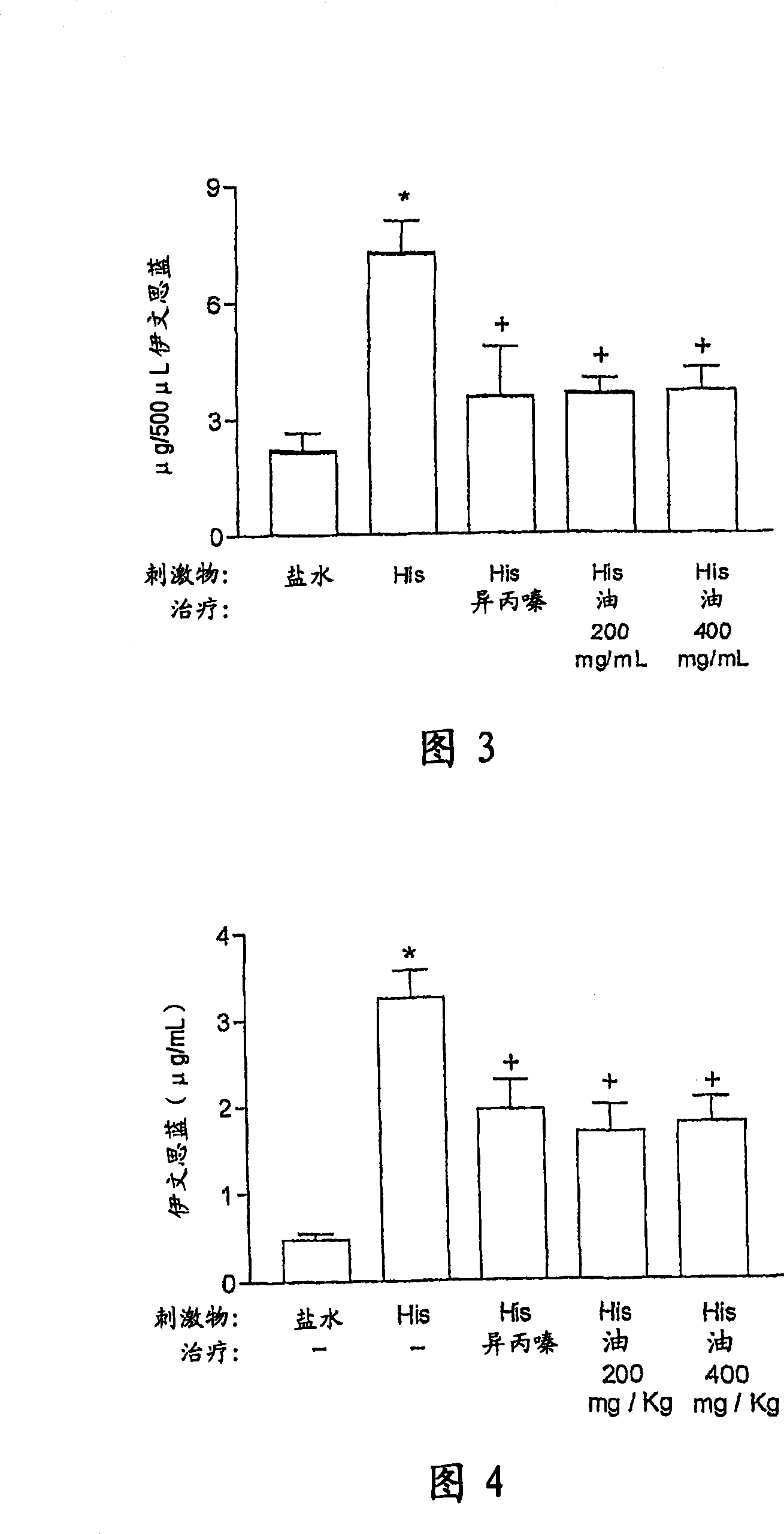
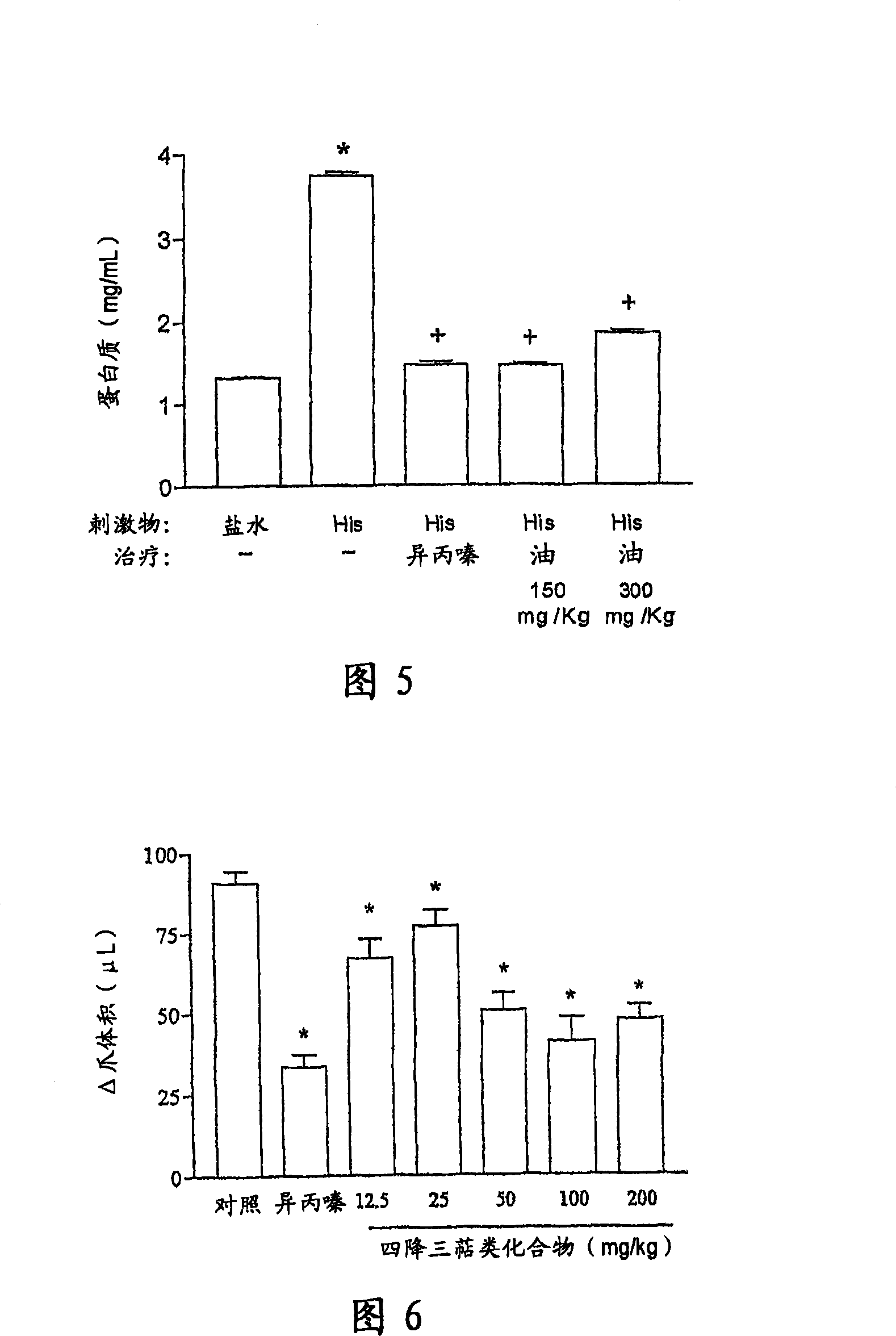
[0167] a) Experiment of paw edema
[0168] Animals were challenged by intraplantar injections of stimulants (histamine, bradykinin and PAF-pl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com