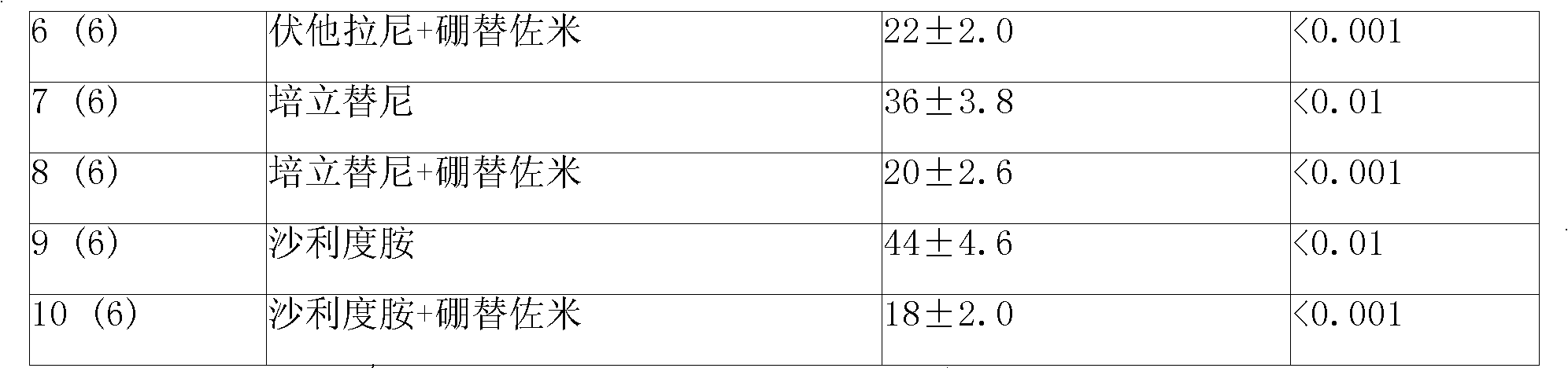Anticancer composition containing neovascularization inhibitor and bortezomib
A technology of bortezomib and neovascularization, which is applied in the direction of active ingredients of boron compounds, drug combinations, non-active ingredients of polymer compounds, etc., and can solve problems such as toxic reactions, inability to effectively kill tumor cells, and burst release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
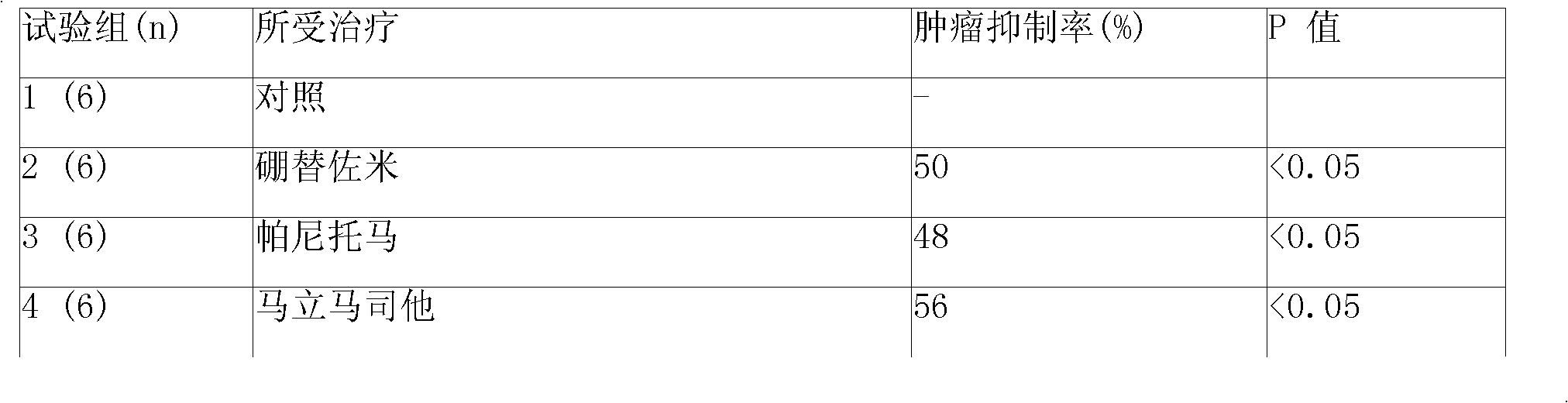
Image
Examples
Embodiment 1
[0108] Put 90, 90 and 80mg p(BHET-EOP / TC), BHET-EOP: TC is 80:20) copolymer into three containers of A, B and C respectively, and then add 100 ml of dichloromethane to each , after dissolving and mixing, add 10mg erlotinib, 10mg bortezomib, 10mg erlotinib and 10mg bortezomib respectively, reshake and prepare 10% erlotinib, 10% boron Tezomib, and microspheres for injection with 10% erlotinib and 10% bortezomib. Then suspend the microspheres in physiological saline containing 15% mannitol to prepare the corresponding suspension-type sustained-release injection. The release time of the slow-release injection in physiological saline in vitro is 40-50 days, and the release time in mice subcutaneously is more than 50 days.
Embodiment 2
[0110] The method step of being processed into slow-release injection is identical with embodiment 1, but difference is that used auxiliary material is the p(BHET-EOP / TC) of 50: 50, containing anticancer active ingredient and weight percent thereof are:
[0111] (1) 5-30% erlotinib or gefitinib;
[0112] (2) 5-30% bortezomib; or
[0113] (3) Combination of 5-30% erlotinib or gefitinib and 5-30% bortezomib.
Embodiment 3
[0115] Put 70 mg of p(LAEG-EOP) with a peak molecular weight of 10,000-25,000 into three containers of A, B, and C, respectively, and then add 100 ml of dichloromethane to each, dissolve and mix well, and pour into the three containers respectively Add 30mg gefitinib, 30mg bortezomib, 15mg gefitinib and 15mg bortezomib, re-shake and use spray drying method to prepare 30% gefitinib, 30% bortezomib, 15% gefitinib Microspheres for injection of tinib and 15% bortezomib. The dried microspheres are suspended in physiological saline containing 1.5% sodium carboxymethylcellulose to prepare the corresponding suspension-type sustained-release injection. The drug release time of the sustained release injection in physiological saline in vitro is 50-65 days, and the drug release time in mice subcutaneous is about 60 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com