Insulin nano transdermal patch and preparation method thereof
A technology for insulin and recombinant human insulin, applied in pharmaceutical formulations, medical preparations containing active ingredients, metabolic diseases, etc., can solve the problems of poor water solubility, low absorption and bioavailability of insulin, and achieve improved efficacy and high drug loading. dose, reducing the effect of drug toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
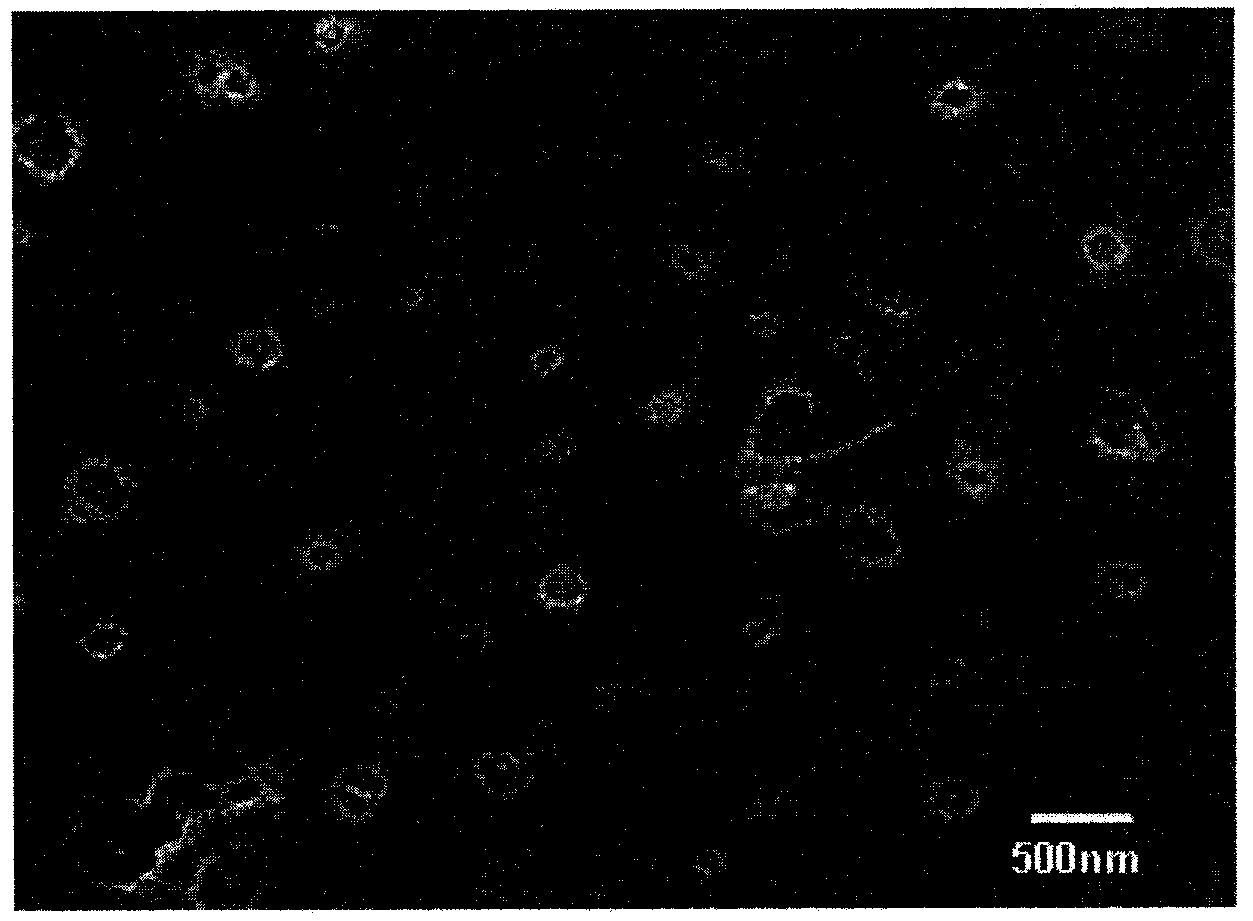
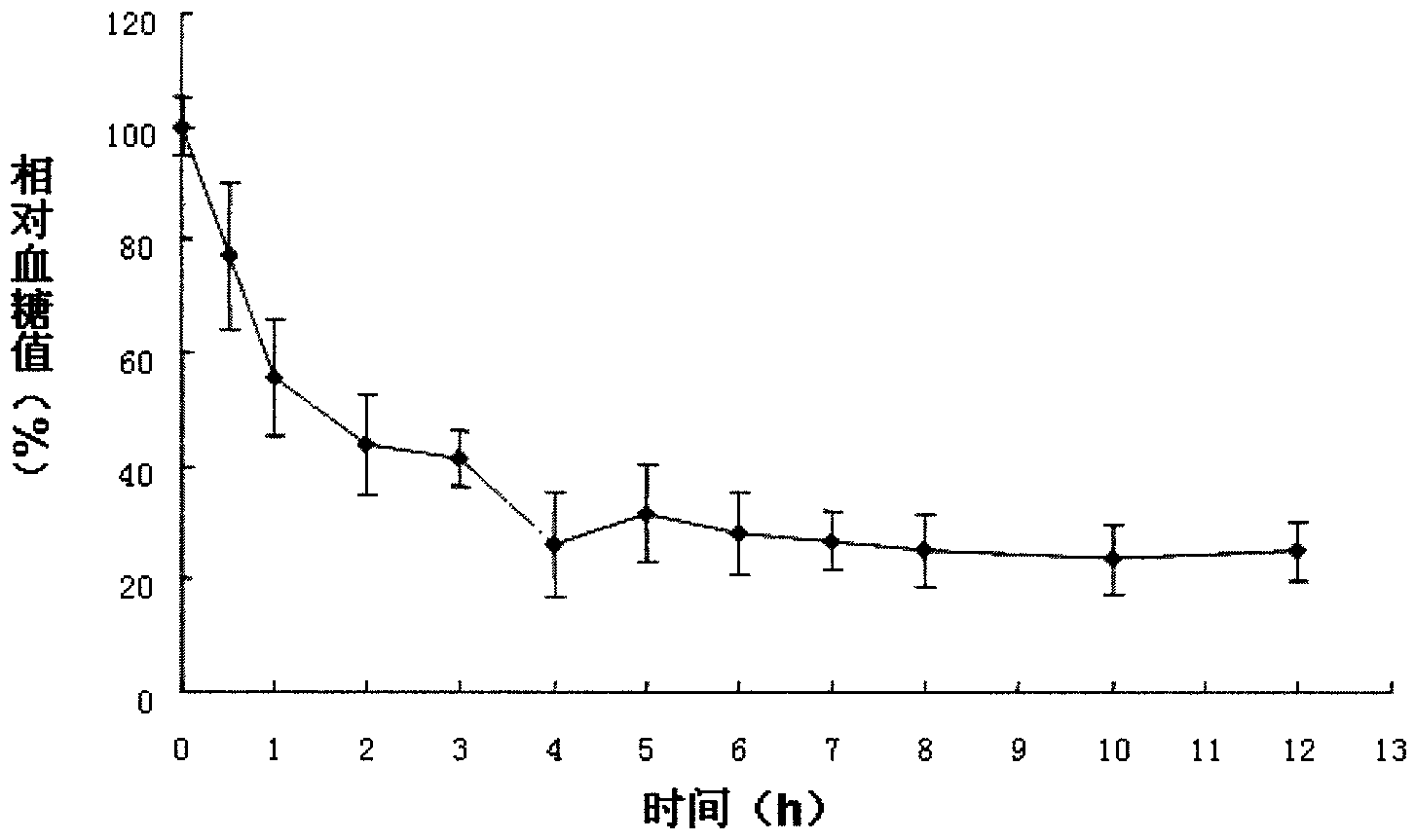
Image
Examples
Embodiment 1
[0026] A: Weigh 8mg of insulin and dissolve it in 10ml of DMF to form an organic solution containing medicine, 300mg of soybean lecithin, 50mg of liposome, 50mg of sodium deoxycholate, 0.1ml of glycerin, 200mg of hypromellose, 0.01M glycine buffer 30ml (pH6.2), 1g of mannitol was dissolved by adding water to 200ml;
[0027] B: The insulin solution is slowly poured into the aqueous solution, stirred, and probe-type ultrasonic to obtain the insulin nanosuspension;
[0028] C: Insulin nano-suspension was freeze-dried at -60°C for 48 hours in a vacuum state to obtain insulin nano-lyophilized powder;
[0029] D: Insulin nano freeze-dried powder, chitosan 50mg, carbomer 5mg, polyethylene glycol 10mg, soybean lecithin 200mg, azone 0.8g, glycerin 0.8g, propylene glycol 0.4g, polyacrylate pressure-sensitive adhesive Mix 4g (dissolved in dichloromethane) with 1g of acrylic pressure-sensitive adhesive and dilute to 5ml with glycine buffer. Stir evenly to obtain a drug-loaded layer;
[...
Embodiment 2
[0032] A: Weigh 8mg of insulin and dissolve it in 10ml of DMF to form a drug-containing organic solution, soybean lecithin 300mg, liposome 30mg, sodium oleate 30mg, glycerin 0.1ml, hypromellose 100mg, glycine buffer 30ml (pH6 .2), 800mg of mannitol is dissolved by adding water to 200ml;
[0033] B: The insulin solution is slowly poured into the aqueous solution, stirred, and probe-type ultrasonic to obtain the insulin nanosuspension;
[0034] C: Insulin nano-suspension was freeze-dried at -60°C for 48 hours in a vacuum state to obtain insulin nano-lyophilized powder;
[0035] D: Insulin nano freeze-dried powder, sodium deoxycholate 50mg, chitosan 50mg, polyethylene glycol 20mg, soybean lecithin 100mg, azone 1g, glycerin 0.8g, propylene glycol 0.4g, polyacrylate pressure-sensitive adhesive Mix 4g (dissolved in dichloromethane) with 1g of acrylic pressure-sensitive adhesive and dilute to 5ml with glycine buffer. Stir evenly to obtain a drug-loaded layer;
[0036] E: The drug-l...
Embodiment 3
[0038] A: Weigh 8mg of insulin and dissolve it in 10ml of DMF to form a drug-containing organic solution, soybean lecithin 300mg, liposome 30mg, oleic acid 2mg, sodium oleate 30mg, hypromellose 80mg, glycine buffer 30ml (pH6 .2), 1g of mannitol is dissolved by adding water to 200ml;
[0039] B: The insulin solution is slowly poured into the aqueous solution, stirred, and probe-type ultrasonic to obtain the insulin nanosuspension;
[0040] C: Insulin nano-suspension was freeze-dried at -60°C for 48 hours in a vacuum state to obtain insulin nano-lyophilized powder;
[0041] D: Insulin nano freeze-dried powder, sodium deoxycholate 30mg, chitosan 30mg, polyethylene glycol 20mg soybean lecithin 150mg, azone 1g, glycerin 0.8g, propylene glycol 0.4g, polyacrylate pressure-sensitive adhesive 4g (dichloromethane dissolved) mixed with 1g of acrylic pressure-sensitive adhesive and diluted to 5ml with glycine buffer solution. Stir evenly to obtain a drug-loaded layer;
[0042] E: The dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com