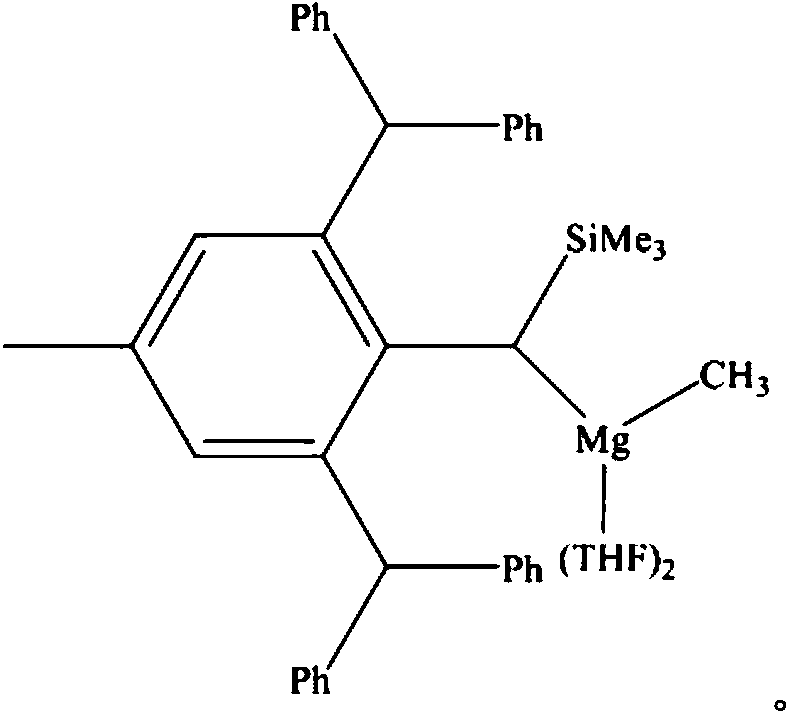
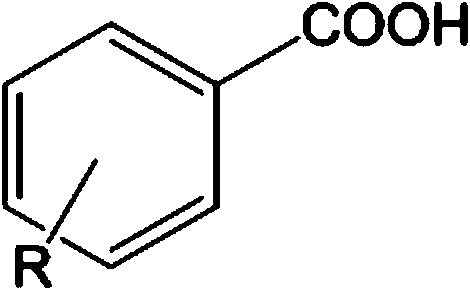
Carboxylic acid deoxidation hydroboration method
A hydroboration and carboxylic acid technology, which is applied in the field of catalytic reaction of magnesium metal compounds, achieves the effects of mild conditions, low catalyst consumption, and expanded application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Large sterically hindered aminomagnesium alkyl compounds catalyze the synthesis of boronate compounds from benzoic acid and borane:
[0025] Under anhydrous and oxygen-free conditions, under the protection of nitrogen, in a glove box, add 0.25 mmol of benzoic acid into a Schlenk reaction flask with a stirring bar, then add 1.0 mmol of pinacol borane and mix well, and finally add a catalyst with a large space 0.00025 mmol of magnesium alkyl compounds stabilized by hindered amino ligands, stirred and reacted at 60°C for 1 hour, briefly pumped under the vacuum pump, and added CDCl 3 Measure NMR. Calculated 1 H spectrum yield 99%. NMR data of the main product: 1 H NMR (600MHz, CDCl 3 ): δ1.25 (s-overlap, 36H, CH 3 , OBpin&pinBOBpin), 4.91(s, 2H, OCH 2 ), 7.23-7.34 (m, 5H, ArH).
Embodiment 2
[0027] Large sterically hindered aminomagnesium alkyl compound catalyzes the synthesis of boronate compounds from p-toluic acid and borane:
[0028] Under anhydrous and oxygen-free conditions, under nitrogen protection, in a glove box, add 0.25 mmol of p-toluic acid to a Schlenk reaction flask with a stirring bar, then add 1.0 mmol of pinacol borane and mix well, and finally add the catalyst 0.00025 mmol of magnesium alkyl compound stabilized by large sterically hindered amino ligands, stirred and reacted at 60°C for 1 hour, briefly pumped under vacuum pump, and added CDCl 3 Measure NMR. Calculated 1 H spectrum yield 98%. NMR data of the main product: 1 H NMR (600MHz, CDCl 3 ): δ1.25 (s-overlap, 36H, CH 3 , OBpin&pinBOBpin), 2.32(s, 3H, ArCH 3 ), 4.87 (s, 2H, OCH 2 ), 7.12(d, 3 J HH =7.8Hz, 2H, ArH), 7.22(d, 3 J HH = 7.8 Hz, 2H, ArH).
Embodiment 3
[0030] Large sterically hindered aminomagnesium alkyl compound catalyzes the synthesis of boronate compounds from p-tert-butylbenzoic acid and borane:
[0031] Under anhydrous and oxygen-free conditions, under nitrogen protection, in a glove box, add 0.25 mmol of p-tert-butylbenzoic acid to a Schlenk reaction flask with a stirring bar, then add 1.0 mmol of pinacol borane and mix well, and finally add Catalyst 0.00025 mmol of magnesium alkyl compound stabilized by large sterically hindered amino ligands, stirred and reacted at 60°C for 1 hour, briefly pumped under vacuum pump, and added CDCl 3 Measure NMR. Calculated 1 H spectrum yield 99%. NMR data of the main product: 1 H NMR (600MHz, CDCl 3 ): δ1.26 (s-overlap, 36H, CH 3 , OBpin&pinBOBpin), 1.31(s, 9H, C(CH 3 ) 3 ), 4.89 (s, 2H, OCH 2 ), 7.27(d, 3 J HH = 8.4 Hz, 2H, ArH), 7.35 (m, 2H, ArH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com