Preparation method of descarbamoyl cefuroxime lactone
A technology of acetyl cephalosporin and nitrofloxide, applied in directions such as organic chemistry, can solve the problems of low amount of DCC lactone, inconvenient separation, unfavorable structure analysis and pharmacological research, etc., and achieves the effect of high yield and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
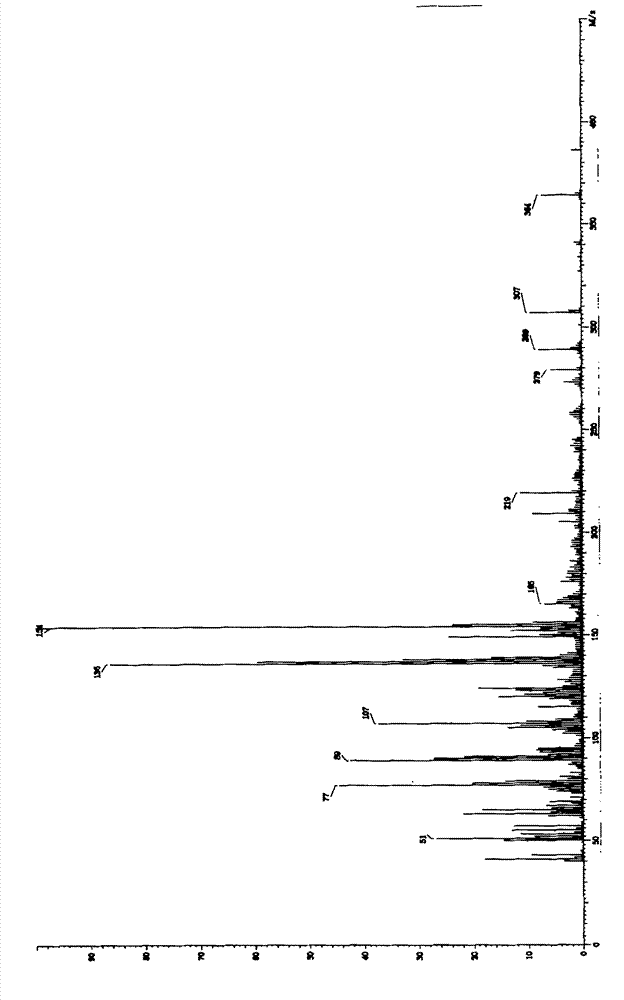
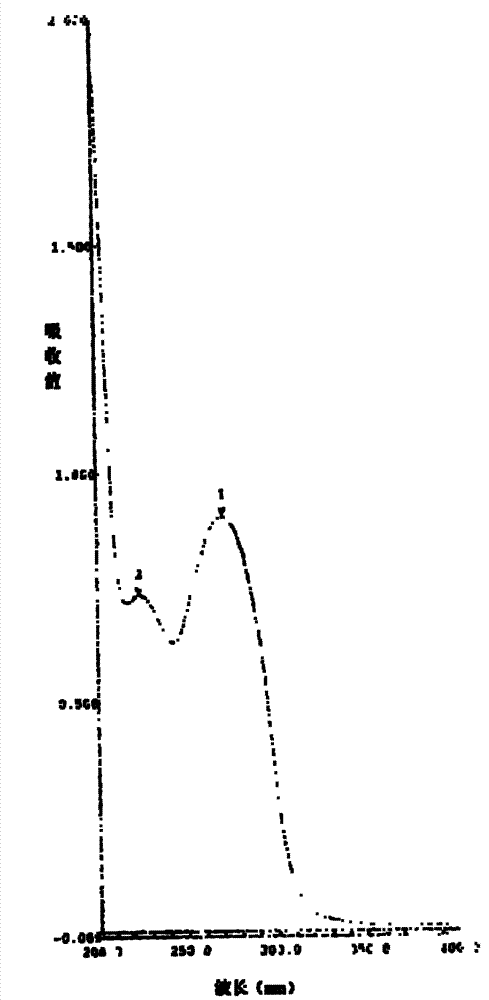
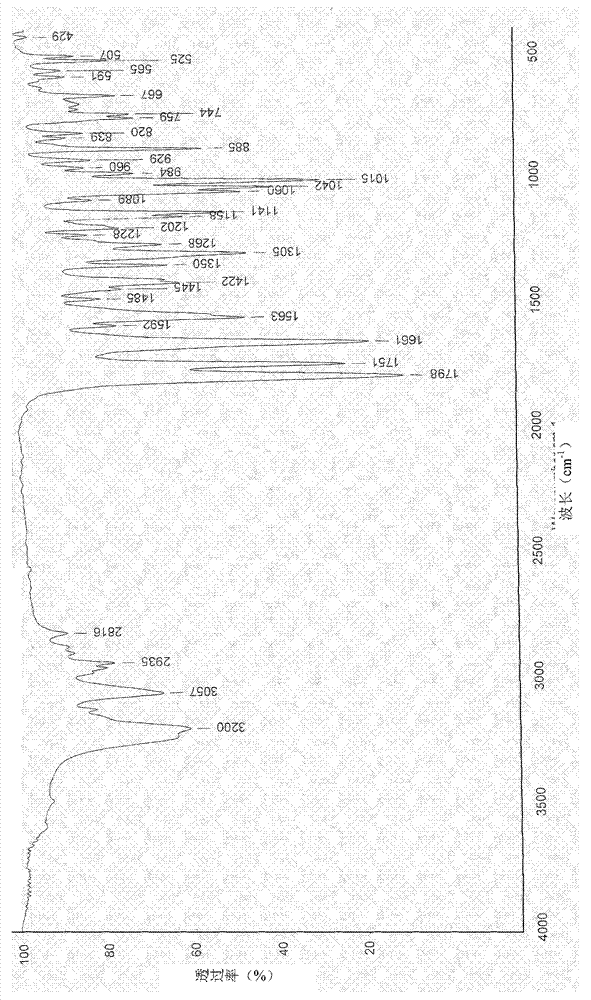
Image
Examples
Embodiment 1
[0028] In a 1000ml four-necked reaction flask, add 70g of DCC and 700ml of acetone, stir well at room temperature (30°C) to dissolve DCC completely, then add 7ml of concentrated sulfuric acid, stir at room temperature (30°C), and a large amount of crystals will precipitate after 10 minutes , maintained stirring, and the reaction was completed after 2.5 hours. The pH value was adjusted to 7.0 with sodium bicarbonate solution, and the precipitate was obtained by filtration, washed successively with acetone, dilute sodium bicarbonate solution and pure water, and then dried at 70°C to obtain 50 g of the target product. The purity (HPLC) of the target product obtained in this embodiment is 99.6%, and the moisture is lower than 1.0%.
Embodiment 2
[0030] In a 1000ml four-necked reaction flask, add 70g of DCC and 700ml of acetonitrile, fully stir at 60°C to dissolve the DCC completely, then add 10.5ml of concentrated hydrochloric acid, reflux and stir at 60°C, a large amount of crystals will precipitate after 5 minutes, keep stirring, After 2 hours the reaction was complete. The pH value was adjusted to 7.2 with sodium bicarbonate solution, and the precipitate was obtained by filtration, washed successively with acetone, dilute sodium bicarbonate solution and pure water, and then dried at 70°C to obtain 60 g of the target product. The purity (HPLC) of the target product obtained in this embodiment is 99.6%, and the moisture is lower than 1.0%.
Embodiment 3
[0032] In a 1000ml four-necked reaction flask, add 70g of DCC, 700ml of DMF and 70ml of acetonitrile, fully stir at 40°C to dissolve the DCC completely, then add 9ml of nitric acid, stir at 40°C, a large amount of crystals are precipitated after 10 minutes, keep stirring, After 2.5 hours the reaction was complete. The pH value was adjusted to 7.0 with sodium bicarbonate solution, and the precipitate was obtained by filtration, washed successively with acetone, dilute sodium bicarbonate solution and pure water, and then dried at 70°C to obtain 54 g of the target product. The purity (HPLC) of the target product obtained in this example is 99.7%, and the moisture is lower than 1.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com