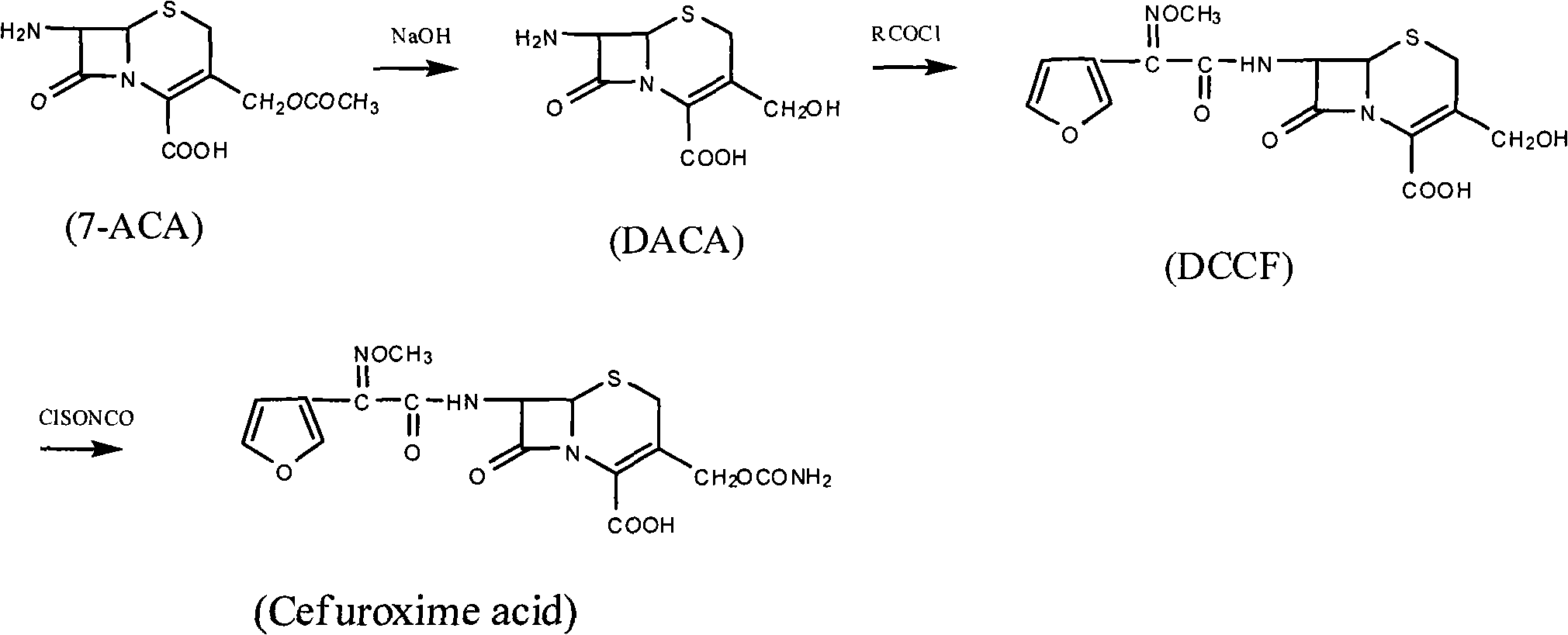
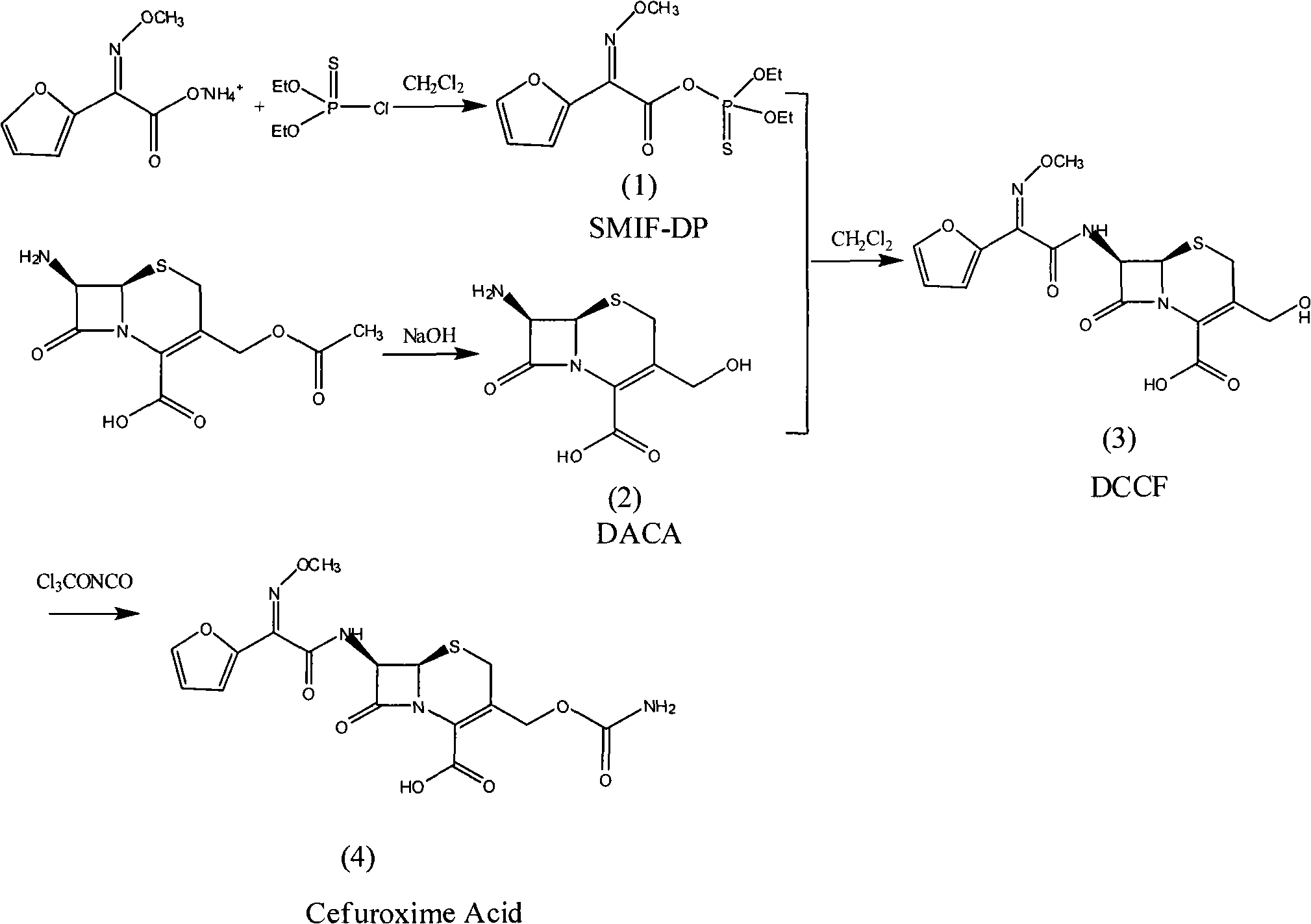
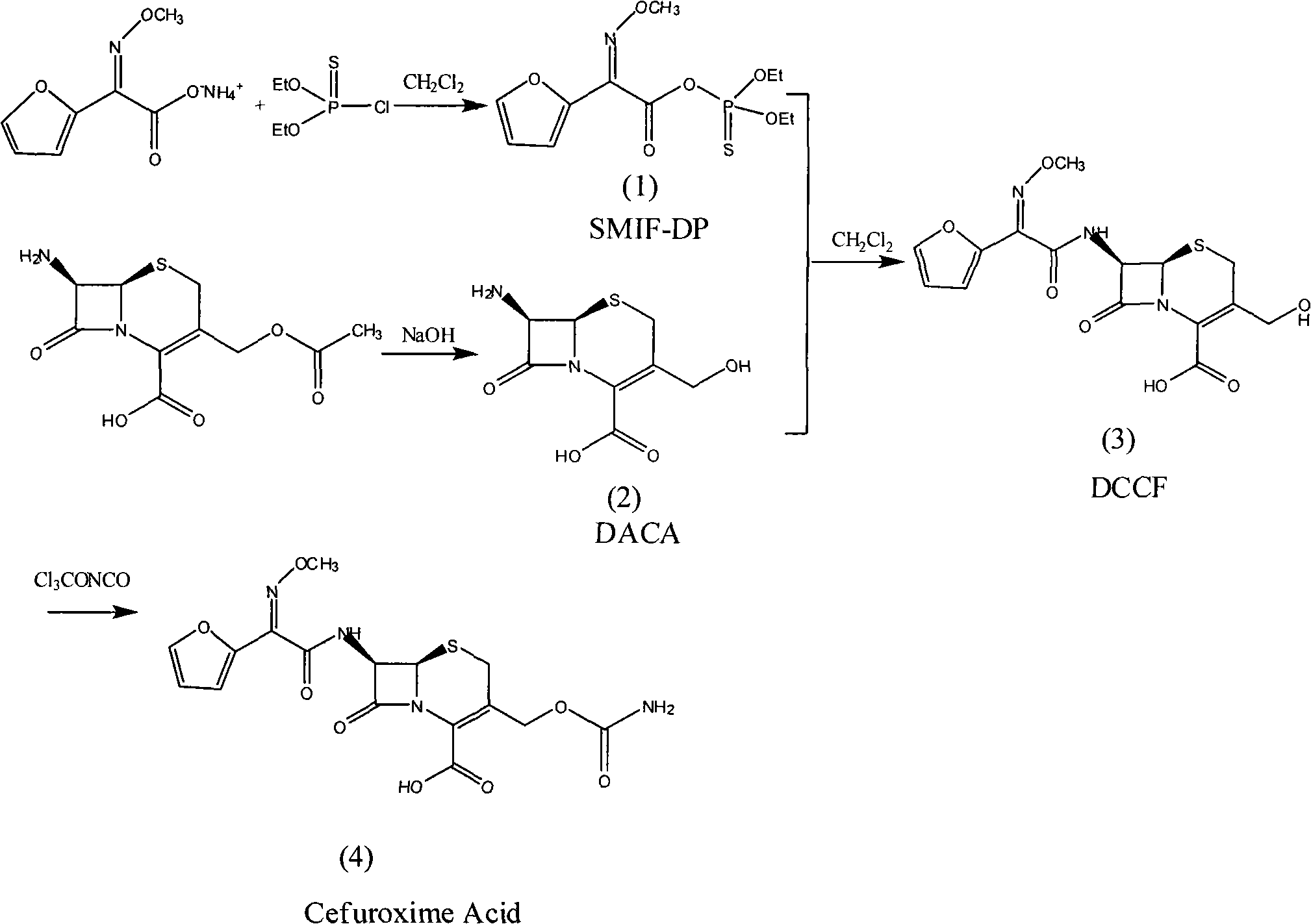
Method for synthesizing cefuroxime acid
A technology of cefuroxime acid and a synthesis method, applied in the field of chemistry, can solve problems such as difficult availability of raw materials, side reactions, dark product color, etc., and achieve the effects of saving one-step reaction, improving yield, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0050] (1) Preparation of SMIF-DP
[0051] In a 250ml four-necked flask, add (Z)-2-(2-furyl)-2-methoxyimino ammonium acetate (SMIA) 23.4g (0.125mol), dissolve in 75mlCH 2 Cl 2 Then add 6.5g (0.035mol) of tributylamine, stir and cool down to 0°C, put 25.5g (0.13mol) diethoxyphosphoryl thiochloride into the dropping funnel, dropwise add within 30min, continue the heat preservation reaction for 2 hour, HPLC detects that all SMIA reactions are complete, wash with water twice, each time with 25ml of water, stir and wash for 20min, separate liquids, and continue to use 50ml of 3% NaHCO at 0°C for the organic phase 3 aqueous solution and 50ml saturated brine, separated, the organic phase was dried with anhydrous magnesium sulfate, and filtered to obtain a light yellow organic solution compound (1) diethoxyphosphorylthio (Z)-2-furyl-2- Methoxyiminoacetate (SMIF-DP), without separation, the purity of the product detected by HPLC is 98%, and it is stored at low temperature for future ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com