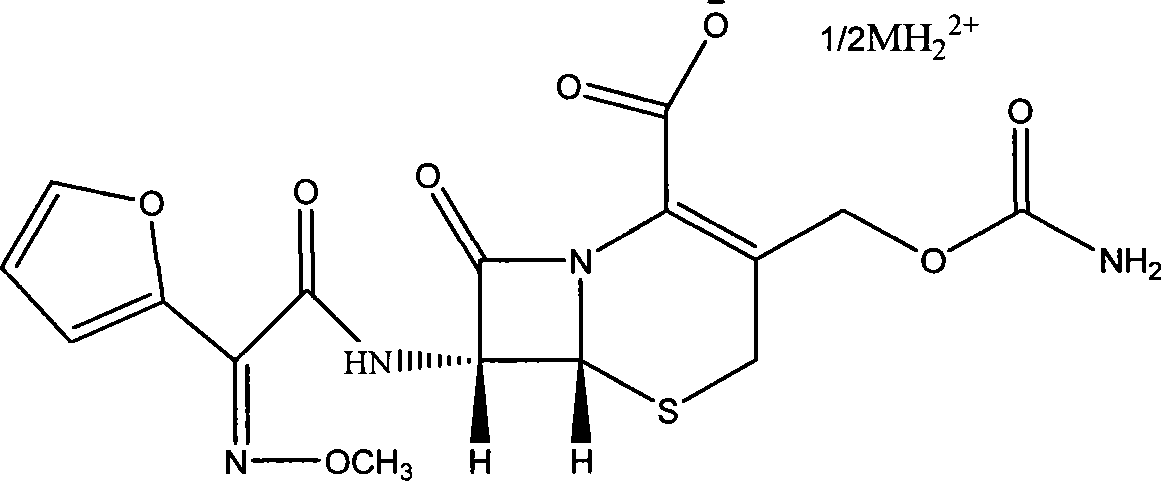
Cefuroxime dibenzyl ethylenediamine salt and preparation method and application thereof
A technology of furoxin dibenzylethylenediamine salt and dibenzylethylenediamine is applied in the field of cefuroxime dibenzylethylenediamine salt and its preparation and application, and can solve the problem of cefuroxime dibenzylethylenediamine salt which has not been reported. Benzylethylenediamine salt and other problems, achieve good commercial value, improve quality, and solve the effect of poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
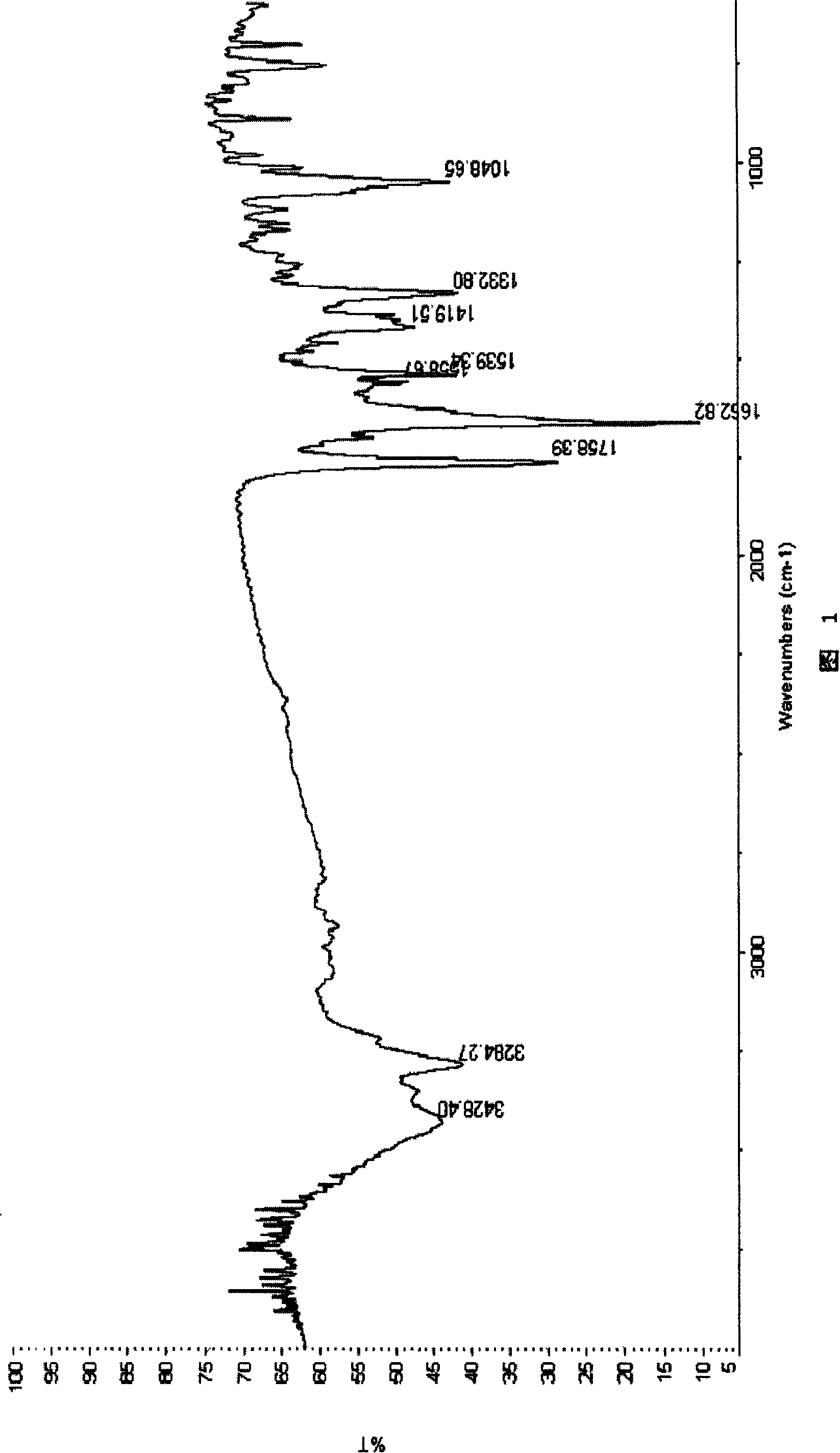
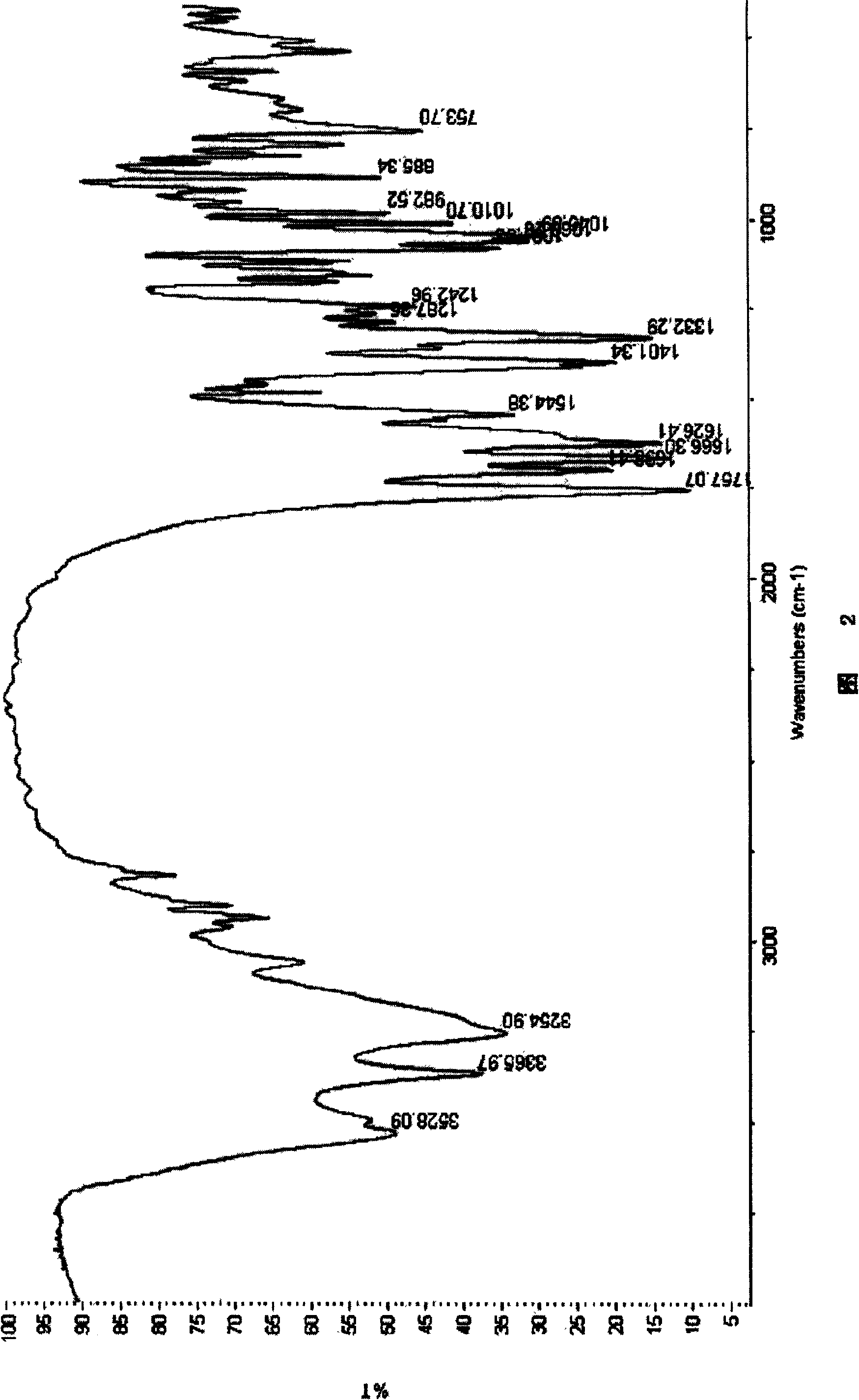
Image
Examples
Embodiment 1
[0046] N, N'-dibenzylethylenediamine-7-[2-furyl (methoxyimino) acetamido] 3-carbamoyloxymethyl-8-oxo-5-thia-1-nitrogen Preparation of heterobicyclo[4.2.0]oct-2-ene-2-carboxylate.
[0047] At room temperature, dissolve 100 g of crude cefuroxime sodium in pure water, stir to make a 10% aqueous solution, adjust the pH of the solution to 2.0-5.0 with sodium acetate, add 100 ml of 95% ethanol, and add N, N' under rapid stirring -Dibenzylethylenediamine diacetate 48g, after adding, continue to stir the reaction mixture for 30 minutes, produce a large amount of white solid, continue to stir for 2 hours at 5~10 ℃, filter, white solid with 5% ethanol solution ( 3×200ml) for washing. Vacuum drying at 40-45° C. gave 116.0 g of white solid cefuroxime dibenzylethylenediamine salt.
Embodiment 2
[0049] N, N'-dibenzylethylenediamine-7-[2-furyl (methoxyimino) acetamido] 3-carbamoyloxymethyl-8-oxo-5-thia-1-nitrogen Preparation of heterobicyclo[4.2.0]oct-2-ene-2-carboxylate.
[0050] At room temperature, dissolve 100 g of crude cefuroxime sodium in pure water, stir to make a 10% aqueous solution, adjust the pH of the solution to 8.0-9.0 with sodium acetate, add 150ml of 95% ethanol, and add N, N' under rapid stirring -Dibenzylethylenediamine diacetate 122g, after adding, continue to stir the reaction mixture for 30 minutes, produce a large amount of white solid, continue to stir for 2 hours at 5~10°C, filter, and the white solid is washed with 5% ethanol solution ( 3×200ml) for washing. Vacuum drying at 40-45° C. gave 118.0 g of cefuroxime dibenzylethylenediamine salt as a white solid.
Embodiment 3
[0052] (6R,7R)-7-[2-furyl(methoxyimino)acetamido]3-carbamoyloxymethyl-8-oxo-5-thia-1-azabicyclo[4.2. 0] Preparation of the sodium salt of oct-2-ene-2-carboxylate.
[0053] Suspend 116.0 g of cefuroxime dibenzylethylenediamine salt in 600 ml of pure water with stirring, cool to 14-16°C, and then add 240 g of Relite CNS (sodium salt). The mixture was stirred at 14-16° C. for 120 minutes until a clear supernatant appeared, and the resin was removed by filtration and washed with 150 ml of 75% ethanol.
[0054] Stir and add 3000ml of acetone to the product solution within 60 minutes at 14-16°C, a white solid precipitates, continue to stir for 30 minutes, filter, wash the crystals with acetone (3×200ml), and dry under vacuum at 40-45°C to obtain cefuroxime Sodium 89.0g, purity 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com