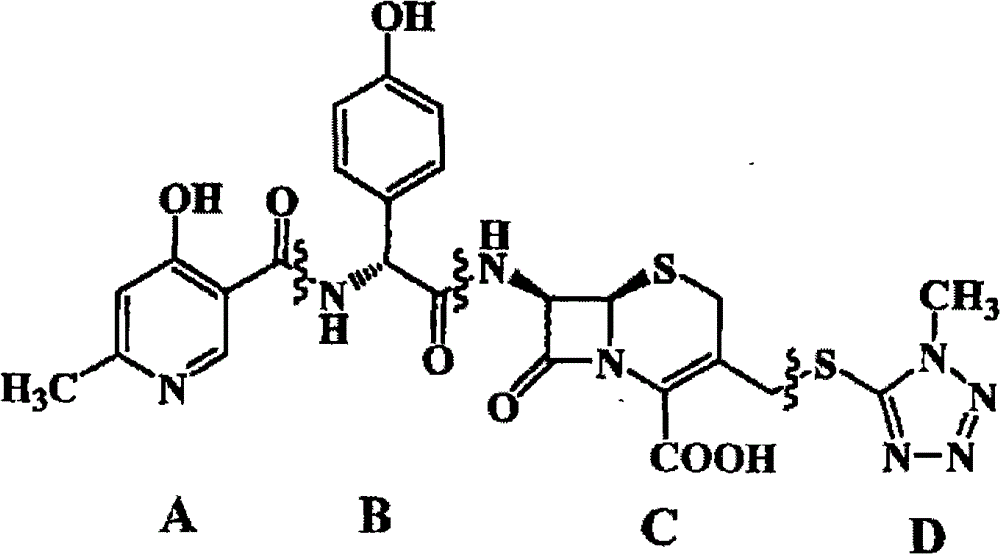
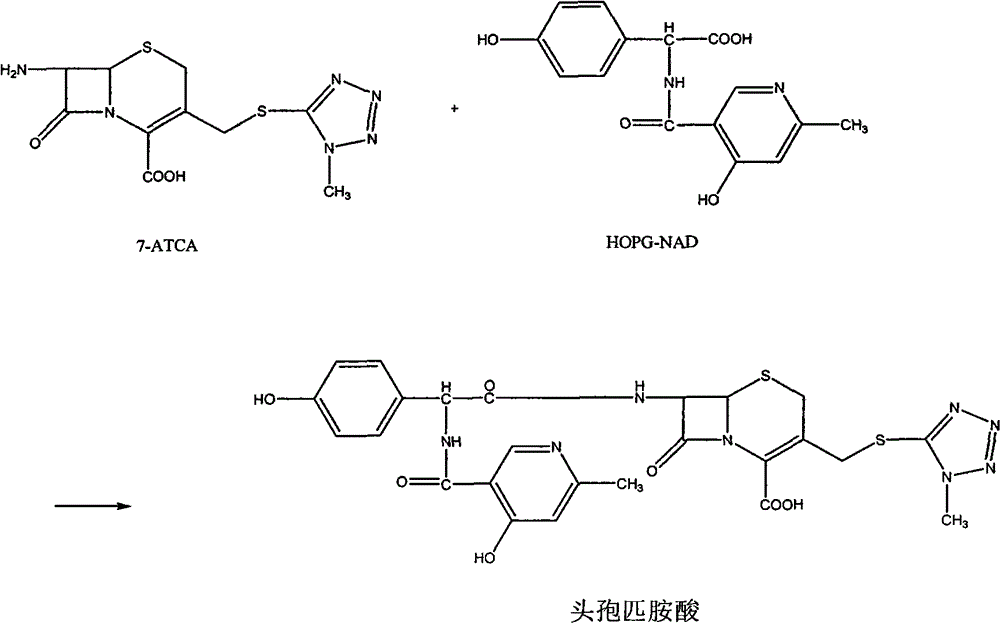
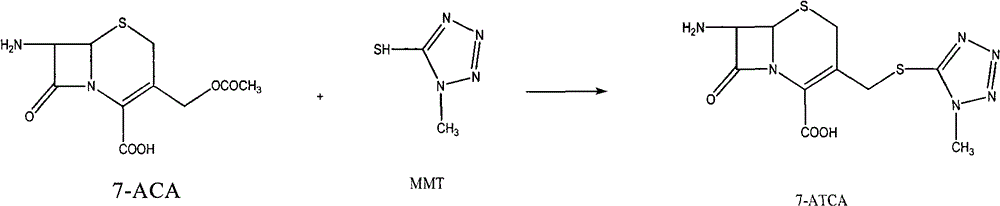
Method for preparing cefpiramide acid
A technology of cefpiramide and carboxylic acid, applied in the direction of organic chemistry and the like, can solve the problems of inability to guarantee the completeness of the protection reaction, unsuitable for large-scale production, easy to produce oil and agglomerate, and achieves overcoming adverse effects, simple and easy operation. control and protection response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This example provides a method for preparing the reactant 7-ATCA used in the method of the present invention.
[0028] Concrete operations are as follows: add 90 grams of 7-aminocephalosporanic acid (7-ACA) in 1000 milliliters of four-necked bottles, 42.1 grams of 1-methyl-1H-tetrazolium-5-mercaptan (MMT), 595 milliliters of acetonitriles, Stir. Add 412.5 ml of boron trifluoride acetonitrile complex (BC) dropwise at room temperature (such as 20-25°C), control the temperature at 28-32°C, and react for 1.5 hours after the addition is complete. 660 ml of purified water, stirred for about 5 minutes to precipitate crystals, stirred slowly for 30 minutes, added dropwise 10% ammonia water to adjust the pH to 2.4-2.6, grown the crystals for 2 hours, filtered, and then used 205 ml of acetonitrile + 90 ml of water, 300 ml mL of water, 300 mL of acetone to wash the crystal. Vacuum-dried at 35-40°C until the water content was less than 1%, to obtain 99 grams of white solid powder...
Embodiment 2
[0037] This example provides a method for preparing the reactant HOPG-NAD-ECF used in the method of the present invention.
[0038]The specific operation is as follows: add 180ml of N,N-dimethylformamide (DMF) to a 250ml three-necked bottle, cool down to below 10°C, add D-α-(6-methyl-4-hydroxynicotinamide)-4-hydroxy Phenylacetic acid (HOPG-NAD) 36g, stirred for 30min, cooled to below -60°C, 20ml of ethyl chloroformate (ECF) was added dropwise for 30min, after the dropwise addition of 13ml of N-methylmorpholine (N-MMP), Stir for 10 minutes after dropping, and cool down to -70°C for later use.
Embodiment 3
[0040] This embodiment provides the method for preparing cefpiramin of the present invention.
[0041] The specific operation is as follows: add 38g of 7-ATCA and 160ml of dichloromethane into a 250ml three-necked flask, add 57ml of BSA dropwise with stirring at room temperature, and stir until the reaction is dissolved; add the obtained reaction solution dropwise into the HOPG-NAD- In the ECF solution, control the temperature at -60°C, react for 4 hours, raise the temperature to 0°C, stir and dropwise add sodium bicarbonate solution (16.7g sodium bicarbonate + 167ml water) for hydrolysis, filter, and let the filtrate stand for stratification, and the organic phase is about 440ml, Add 100ml of water each time, extract twice, combine the obtained aqueous phases to about 360ml, add 360ml of acetone, add hydrochloric acid dropwise to adjust the pH to 1~2 and crystallize, grow the crystals at 0~10°C for 1 hour, filter, and wash with 100ml of water and 100ml of acetone respectively ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com