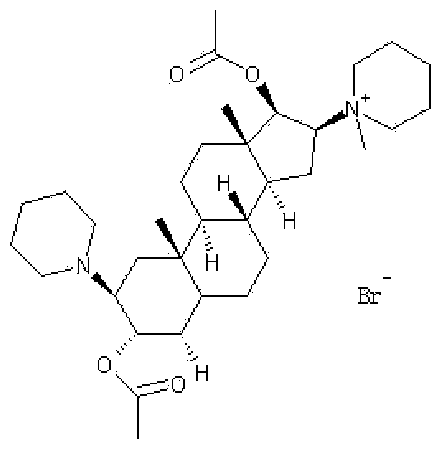
Vecuronium bromide freeze-dried powder injection for injection and preparation method thereof
A technology of freeze-dried powder injection and vecuronium bromide, applied in the field of vecuronium bromide freeze-dried powder injection for injection and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
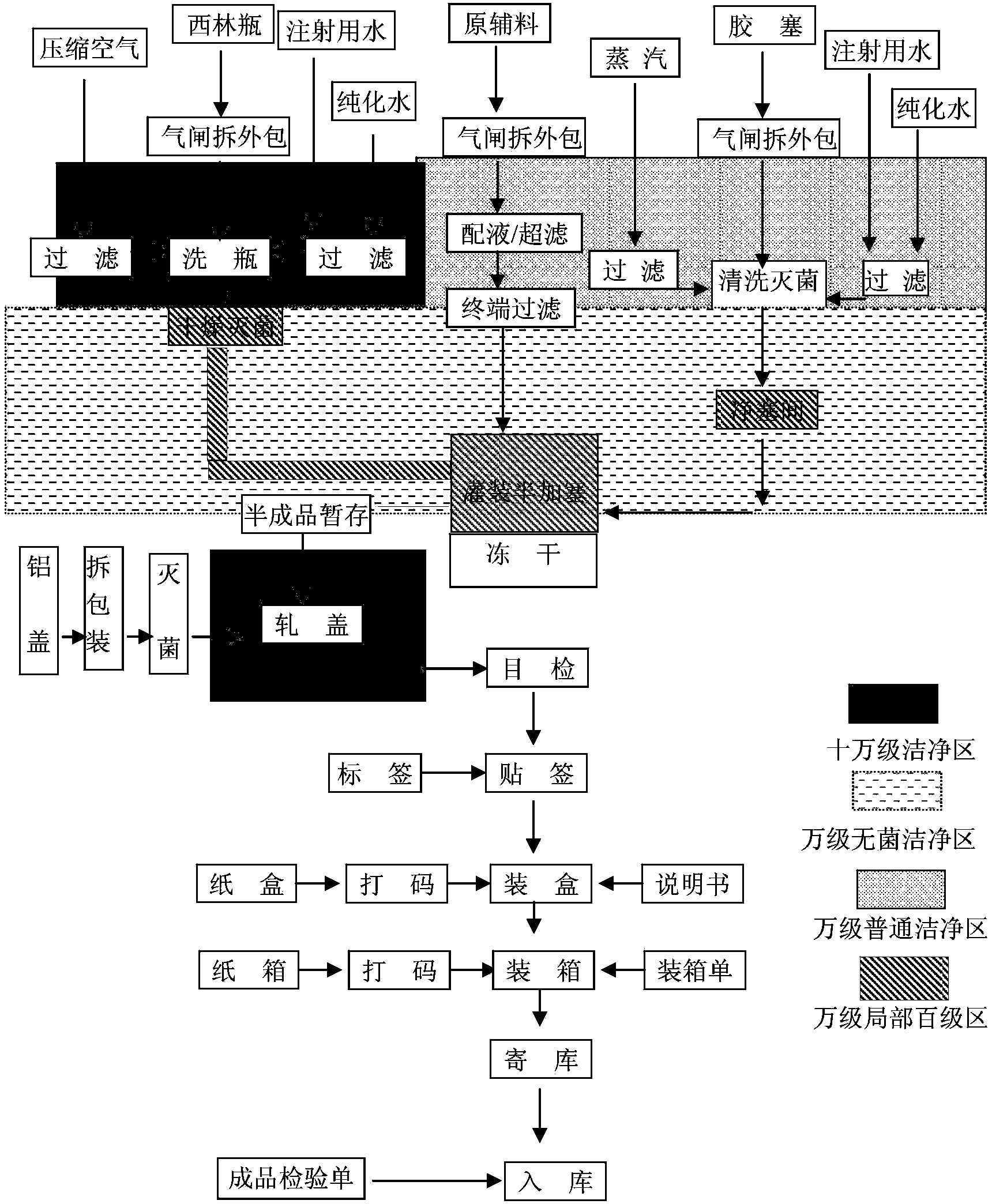
Image
Examples
Embodiment 1
[0027] Embodiment 1, the preparation of vecuronium bromide freeze-dried powder injection for injection
[0028] prescription:
[0029]
[0030]
[0031] The method for preparing the above-mentioned vecuronium bromide freeze-dried powder for injection comprises the following steps:
[0032] Add water for injection at 60°C and 50% of the prescribed amount into the liquid preparation tank, then weigh the prescribed amount of mannitol, lactose, and trehalose into the liquid preparation tank and stir until dissolved, then weigh the prescribed amount of citric acid and phosphoric acid Add disodium hydrogen into the liquid mixing tank and stir until dissolved; then add 40% of the prescribed amount of water for injection, control the temperature at 25-30°C, and weigh the prescribed amount of vecuronium bromide in the dark, add it to the liquid mixing tank and stir to dissolve. After completely dissolving, add water for injection to the full amount, stir evenly, use citric acid ...
Embodiment 2
[0066] Embodiment 2, investigating the influence of different freeze-drying processes on the vecuronium bromide freeze-dried powder injection for injection Prescription: same as embodiment 1.
[0067] Preparation process: Only the freeze-drying process is different from Example 1, and the rest are the same as Example 1.
[0068] 1) Investigate the influence of different pre-freezing time on product properties
[0069]
[0070] 2) Investigate the influence of different pre-freezing methods on product properties
[0071]
[0072]
[0073] 3) Investigate the influence of different sublimation temperature and time on product properties
[0074]
[0075] 4) Investigate the effect of re-drying temperature on product moisture and related substances
[0076] Freeze-dried products generally need to increase the temperature to further remove moisture after the first sublimation drying, but for products with poor stability, increasing the temperature will increase the numbe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com