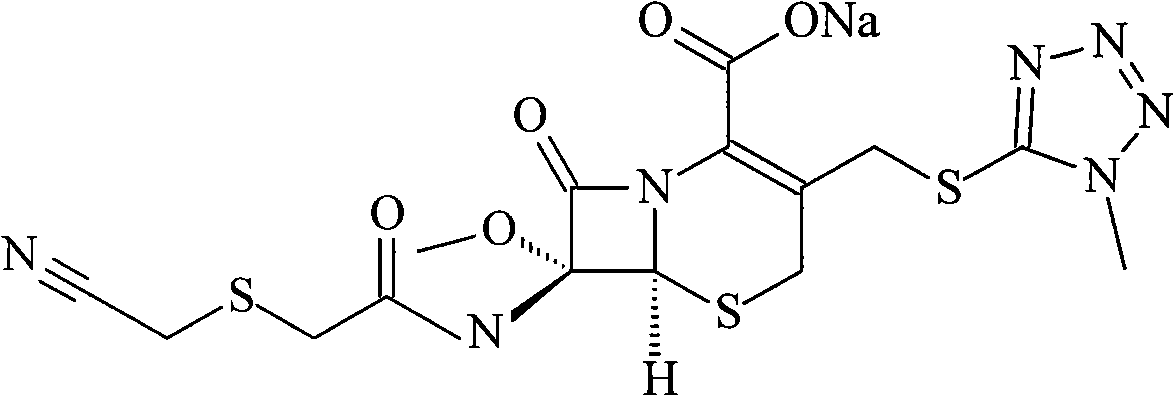
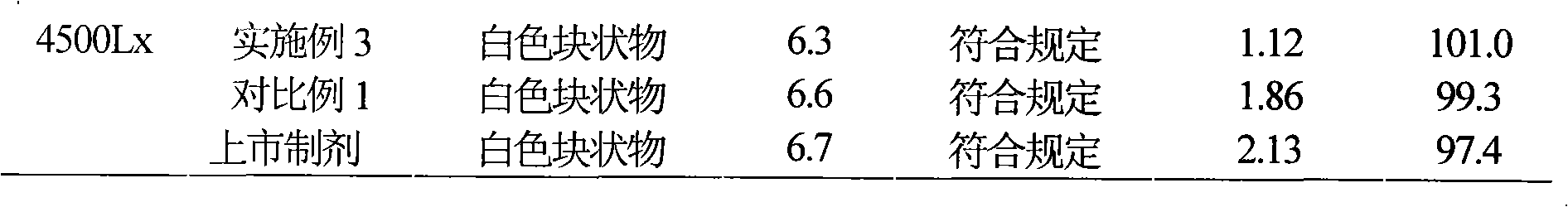Cefmetazole sodium suspension injection powder and novel application thereof
A technology of cefmetazole sodium and powder injection, which is applied in the field of medicine, can solve the problems of low bioavailability and poor stability, and achieve the effects of reducing drug toxicity, not easy to decompose, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
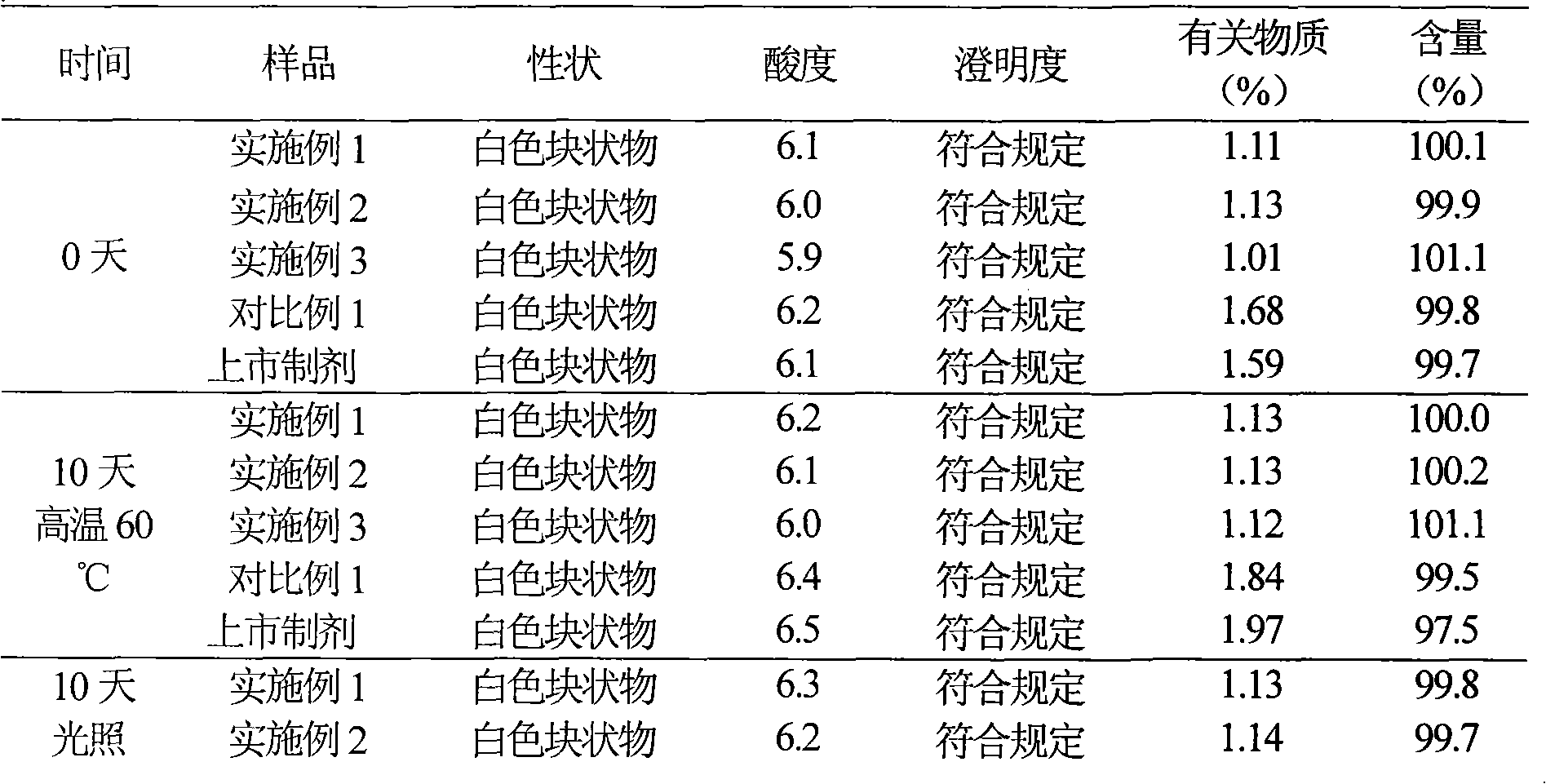
[0042] Example 1 Preparation of Cefmetazole Sodium Suspension Powder Injection
[0043] Prescription: (100 bottles)
[0044] Cefmetazole Sodium 25g
[0045] Chitosan 30g
[0046] Fatty Acid Sorbitan 20 25g
[0047] Sodium cholate 20g
[0049] Ethanol 400ml
[0050] Preparation Process:
[0051] (1) 25 g of cefmetazole sodium was dissolved in 400 ml of water, 30 g of chitosan and 20 g of sodium cholate were added to 400 ml of ethanol for dissolving, then mixed with the aqueous solution and stirred for 30 min to obtain an emulsion;
[0052] (2) 25g fatty acid sorbitan 20 is dissolved in 200ml water for injection;
[0053] (3) The emulsion is evaporated to remove the organic solvent, the fatty acid sorbitan 20 solution in (2) is added in the emulsion, then 23g of sodium chloride is added, after stirring at room temperature, freeze-dry to obtain cefmetazole sodium suspension Powder preparations.
Embodiment 2
[0065] Example 2 Preparation of Cefmetazole Sodium Freeze-dried Suspension Powder Injection
[0066] Prescription: (100 bottles)
[0067] Cefmetazole Sodium 12.5g
[0068] Chitosan 26g
[0069] Polysorbate 80 21.6g
[0070] Glycerin 18g
[0071] Mannitol 16g
[0072] Isopropanol 400ml
[0073] Preparation Process:
[0074] (1) 12.5 g of cefmetazole sodium was dissolved in 350 ml of water, 26 g of chitosan and 18 g of glycerin were added to 400 ml of isopropanol for dissolving, then mixed with the aqueous solution and stirred for 30 min to obtain an emulsion;
[0075] (2) 21.6g polysorbate 80 was dissolved in 200ml water for injection;
[0076] (3) Evaporate the emulsion to remove the organic solvent, add the solution in (2) to the emulsion, add 16g of mannitol, stir evenly at room temperature, freeze-dry to obtain cefmetazole sodium suspension powder preparation.
Embodiment 3
[0077] Example 3 Preparation of Cefmetazole Sodium Freeze-dried Suspension Powder Injection
[0078] Prescription: (100 bottles)
[0079] Cefmetazole Sodium 12.5g
[0080] Gelatin 16g
[0081] Polysorbate 80 14g
[0082] Fatty Acid Sorbitan 20 8g
[0083] Glycerin 18g
[0084] Lactose 10g
[0085] Sodium chloride 26g
[0086] Ethanol 400ml
[0087] Preparation Process:
[0088] (1) Dissolve 12.5 g of cefmetazole sodium in 400 ml of water, add 16 g of gelatin and 18 g of glycerin into 400 ml of ethanol for dissolving, then mix and stir with the aqueous solution for 30 min to obtain an emulsion;
[0089] (2) Dissolve 14g polysorbate 80 and 8g fatty acid sorbitan 20 in 200ml water for injection
[0090] (3) The emulsion is evaporated to remove the organic solvent, the solution in (2) is added to the emulsion, then 26g of sodium chloride is added, after stirring at room temperature, freeze-dry to obtain cefmetazole sodium suspension powder preparation .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com