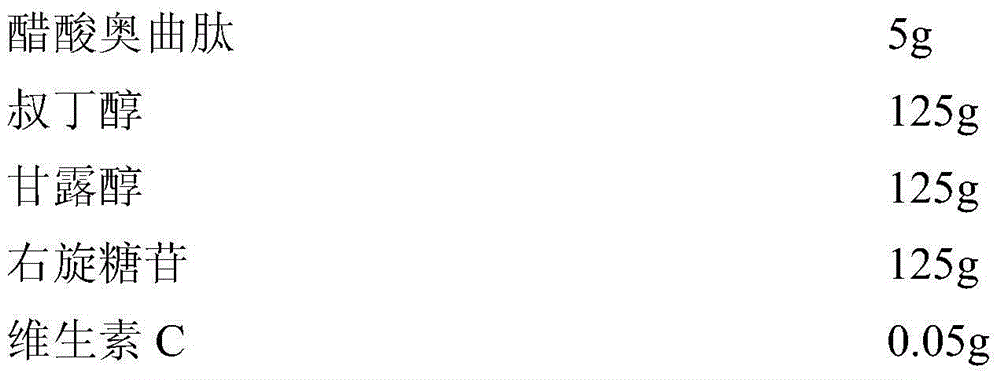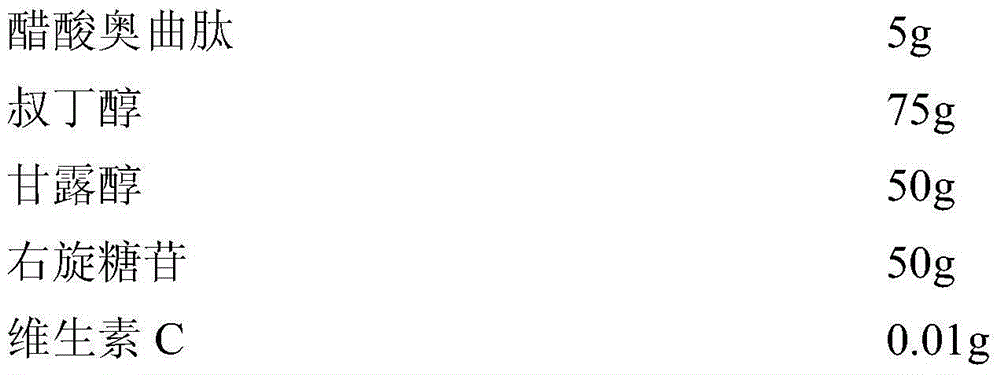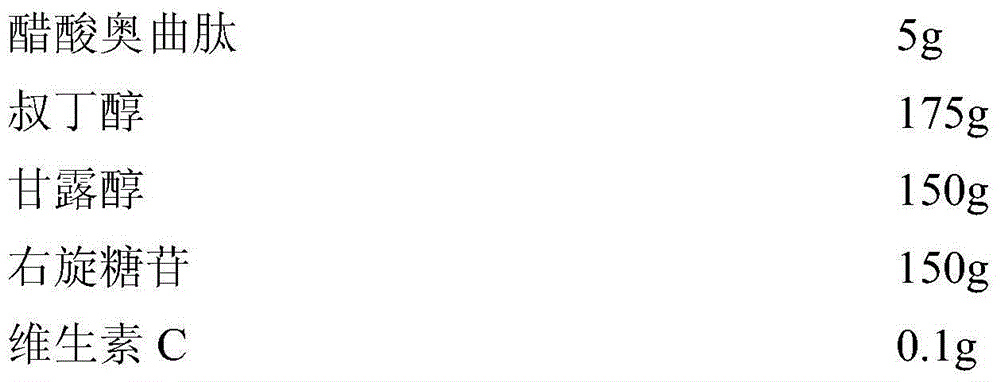Octreotide acetate sterile injection powder preparation and preparation method thereof
A technology for octreotide acetate and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations, can solve the problems of influence, lack of protective measures, microbial contamination and the like, and achieves the effects of simple preparation method, extended shelf life and increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 The preparation of octreotide acetate freeze-dried powder injection of the present invention
[0032] prescription:
[0033]
[0034] Specific steps are as follows:
[0035] 1) Weigh each component according to the formula, add vitamin C, mannitol, dextran, tert-butanol and octreotide acetate into purified water in sequence under nitrogen protection, and stir to dissolve completely;
[0036] 2) filling the prepared medicinal solution into ampoules and freeze-drying.
[0037] 3) Freeze-drying curve: pre-freeze at -35°C for 5 hours; under vacuum conditions -35°C for 5 hours -20°C for 3 hours and then end.
Embodiment 2
[0038] Embodiment 2 The preparation of octreotide acetate freeze-dried powder injection of the present invention
[0039] prescription:
[0040]
[0041] Specific steps are as follows:
[0042] 1) Weigh each component according to the formula, add vitamin C, mannitol, dextran, tert-butanol and octreotide acetate into purified water in sequence under nitrogen protection, and stir to dissolve completely;
[0043] 2) filling the prepared medicinal solution into ampoules and freeze-drying.
[0044] 3) Freeze-drying curve: Pre-freeze at -40°C for 5 hours; under vacuum condition, -40°C for 2 hours, -35°C for 2 hours, and -20°C for 3 hours, then end.
Embodiment 3
[0045] Embodiment 3 The preparation of octreotide acetate freeze-dried powder injection of the present invention
[0046] prescription:
[0047]
[0048] Specific steps are as follows:
[0049] 1) Weigh each component according to the formula, add vitamin C, mannitol, dextran, tert-butanol and octreotide acetate into purified water in sequence under nitrogen protection, and stir to dissolve completely;
[0050] 2) filling the prepared medicinal solution into ampoules and freeze-drying.
[0051] 3) Freeze-drying curve: Pre-freeze at -40°C for 5 hours; under vacuum condition, it lasts at -40°C for 2 hours, at -30°C for 2 hours, and at -20°C for 3 hours before ending.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com