Peramivir impurity A and impurity C, and preparation methods and application thereof
An impurity and reaction technology, applied in the field of preparation of new peramivir impurities, can solve the problems of drug resistance, affecting the effect of vaccine prevention and control, difficult to meet the demand for anti-influenza drugs, etc., and achieve the effect of safety guarantee
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
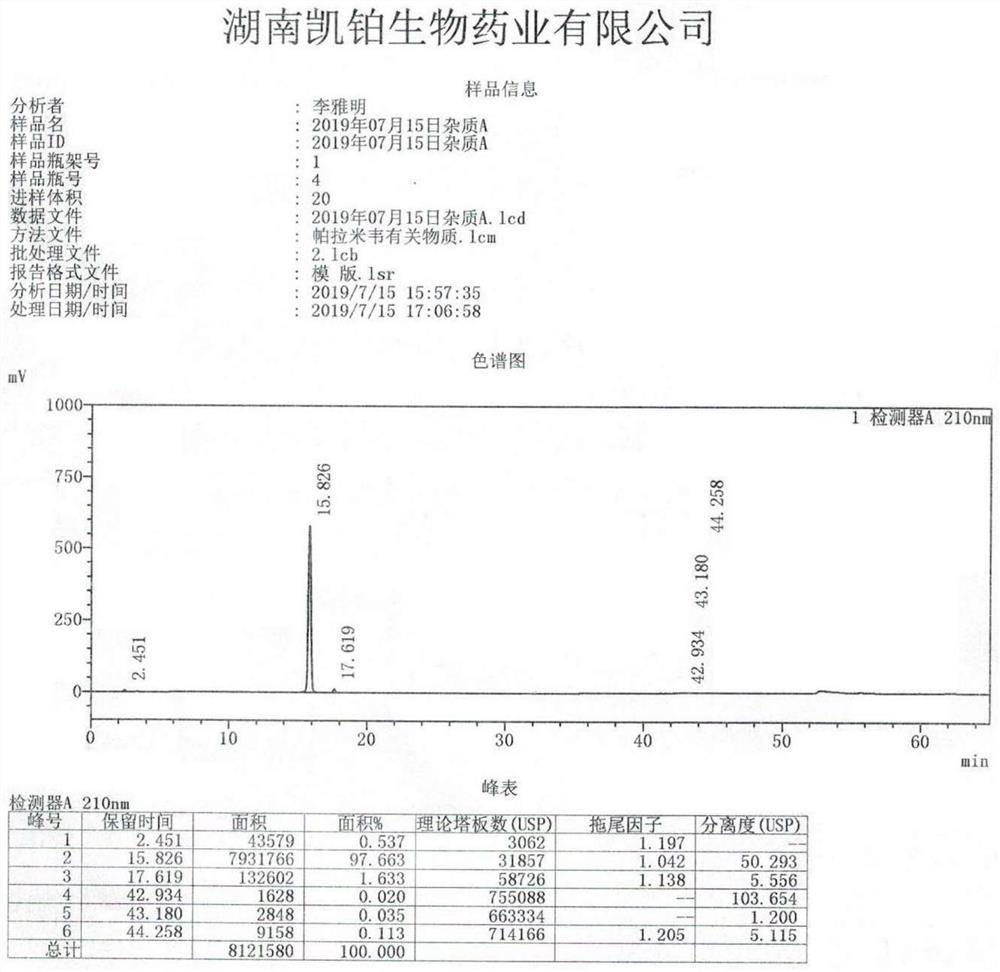
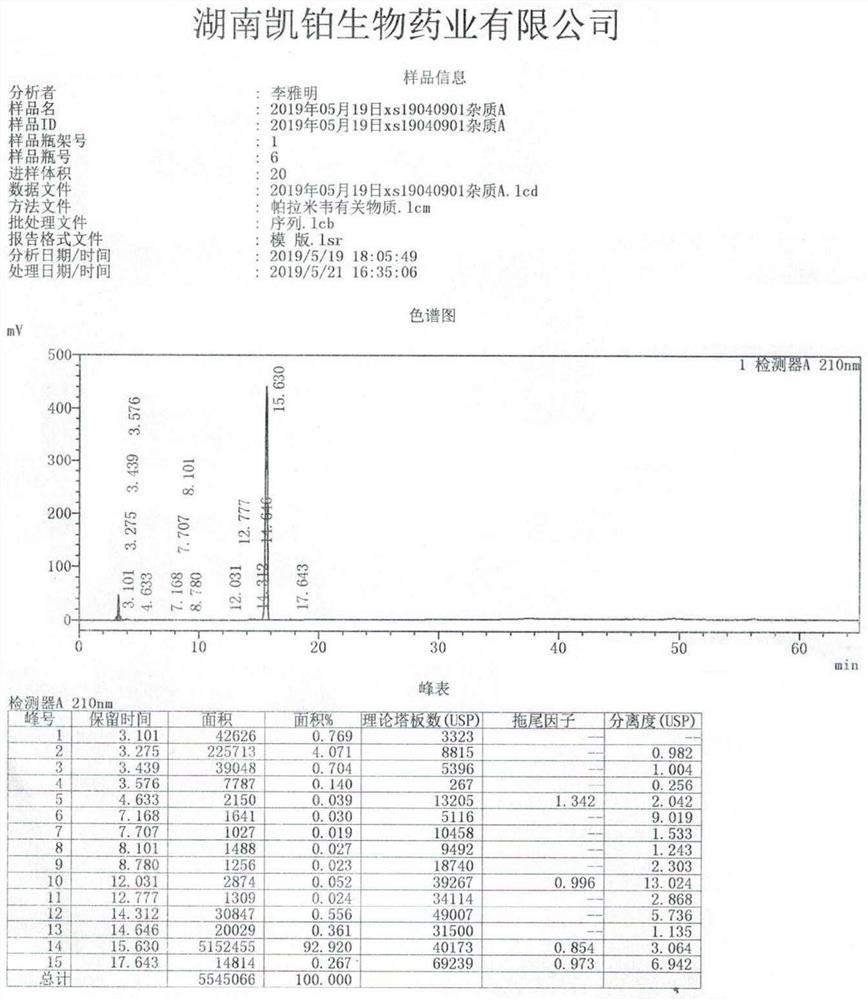
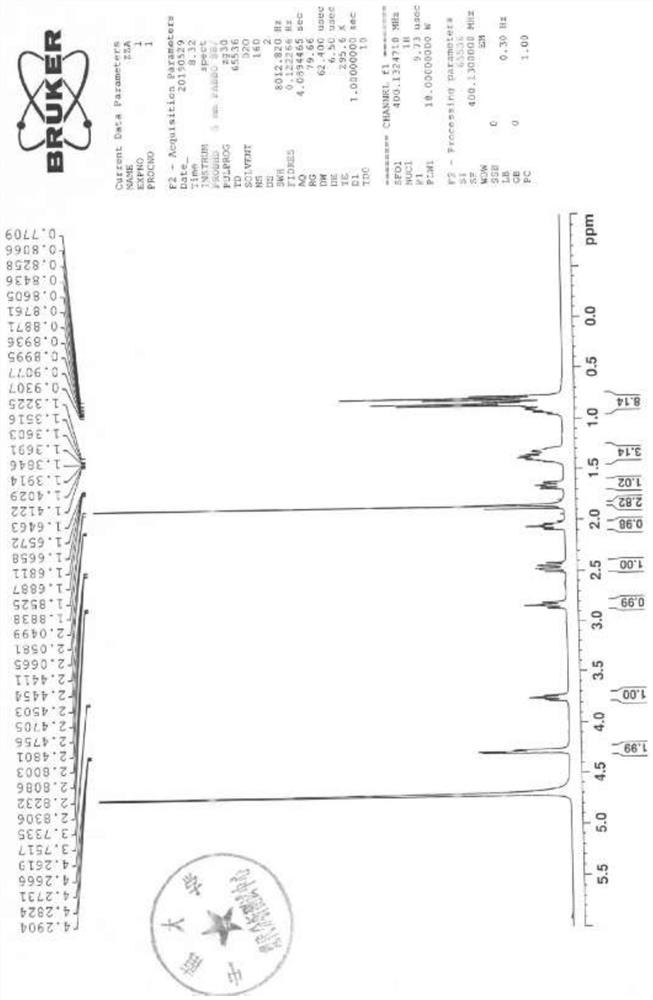
Image
Examples
Embodiment 1
[0061] The preparation of embodiment 1 impurity A
[0062] ① Preparation of 2-ethyl-N-hydroxybutyrimide chloride (compound A-2)
[0063] 3.45 g of hydroxylamine hydrochloride, 3.3 g of water, and 10.5 g of toluene were mixed, and 4.74 g of 2-ethylbutyraldehyde was added with stirring. At 10 to 25°C, 6.95 g of a 30% sodium hydroxide solution (containing 2.085 g of sodium hydroxide) was added over 1 hour. After addition, continue to stir for 1.5h. Stand to separate layers, take the upper toluene layer, directly used in the next step. Suspend 6.32 g of N-chlorosuccinimide (NCS) in 7.5 g of dimethylformamide and cool to 0-10°C. The toluene solution of the above oxime was added in 2.5 hours, and the reaction temperature did not exceed 25°C during the dropwise addition. After the addition was complete, stirring was continued for 1.5 hours. After completion of the reaction, 8.3 g of water was added, stirred for 30 minutes, and the reaction temperature was controlled not to excee...
Embodiment 2
[0074] The preparation of embodiment 2 impurity A
[0075] ① Preparation of 2-ethyl-N-hydroxybutyrimide chloride (compound A-2)
[0076] 3.5 g of hydroxylamine hydrochloride, 3.3 g of water, and 10.5 g of toluene were mixed, and 4.8 g of 2-ethylbutyraldehyde was added with stirring. At 10 to 25°C, 6.90 g of a 30% sodium hydroxide solution (containing 2.0 g of sodium hydroxide) was added over 1 hour. After addition, continue to stir for 1.5h. Leave to stand for stratification, take the upper toluene layer, and use it directly for the next step. Suspend 6.3 g of N-chlorosuccinimide (NCS) in 7.5 g of dimethylformamide and cool to 0-10°C. The toluene solution of the above oxime was added in 2.5 hours, and the reaction temperature did not exceed 25°C during the dropwise addition. After the addition was complete, stirring was continued for 1.5 hours. After completion of the reaction, 8.3 g of water was added, stirred for 30 minutes, and the reaction temperature was controlled n...
Embodiment 3
[0087] The preparation of embodiment 3 impurity C
[0088] ①(1S, 2S, 3S, 4R, 1'S)-3-[(1-amino-2'-ethyl)butyl]-4-[[(1,1-dimethylethoxy)carbonyl]amino Preparation of ]-2-hydroxycyclopentane-1-carboxylic acid methyl ester (compound C-2)
[0089] (3aR, 4R, 6R, 6aS)-4-[[(1,1-dimethylethoxy)carbonyl]amino]-3-(1'-ethylpropyl)-3a,5,6, Dissolve 100g of 6a-tetrahydro-4H-cyclopenta[d]isoxazole-6-carboxylic acid methyl ester (compound C-1) and 30g of anhydrous nickel chloride in 300g of methanol, and cool to -10~5℃ . Dissolve 0.1 g of sodium hydroxide and 33 g of sodium borohydride in formazan (250 g, add to the reaction solution in about 1 hour, and stir the reaction solution at 0-5° C. for 60 minutes. Add 180 g of sodium nitrite, 50 g of ammonium chloride and 25% The solution formed in 60g of ammonia water and 500mL of water. Stir at room temperature for 2 hours. Suction filtration, the filtrate was extracted twice with 800g of dichloromethane, combined with the dichloromethane phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com