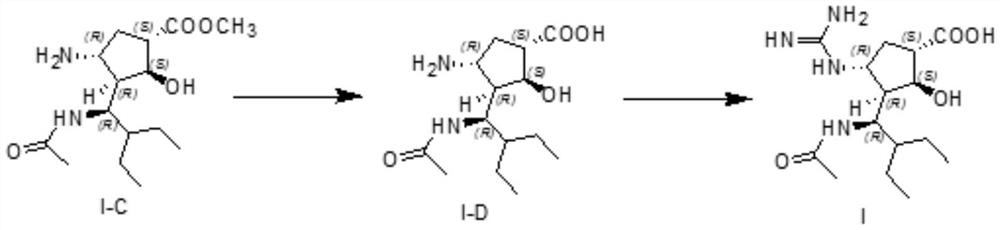
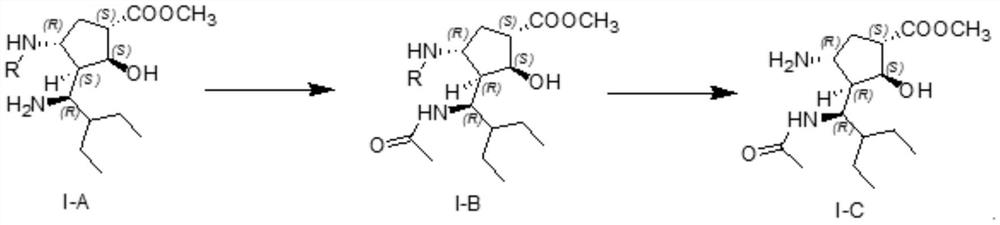
Preparation method and application of peramivir related substances
A technology of related substances and reactions, applied in the field of preparation of peramivir related substances, can solve problems such as affecting the quality of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] 10.0g of the crude product of compound I-A was added to the reaction flask, then 70ml of toluene was added as a reaction solvent, then heated to 48-52°C, and 2.85g of acetic anhydride was added dropwise under temperature control within this temperature range, and the dripping was completed and maintained The temperature of the system was kept between 48-52 °C and the reaction was stirred. After the TLC monitored the disappearance of the raw material reaction, the reaction system was cooled to room temperature, washed once with an aqueous sodium chloride solution, left to stand for liquid separation, and the separated organic phase was then chlorinated again. The sodium aqueous solution was washed once, left to stand for separation, and the obtained organic phase was concentrated to dryness under reduced pressure to obtain 11.08 g of crude I-B.
[0032] The crude I-B was purified by column chromatography with 3% methanol-dichloromethane system to obtain 3.1 g ...
Embodiment 2
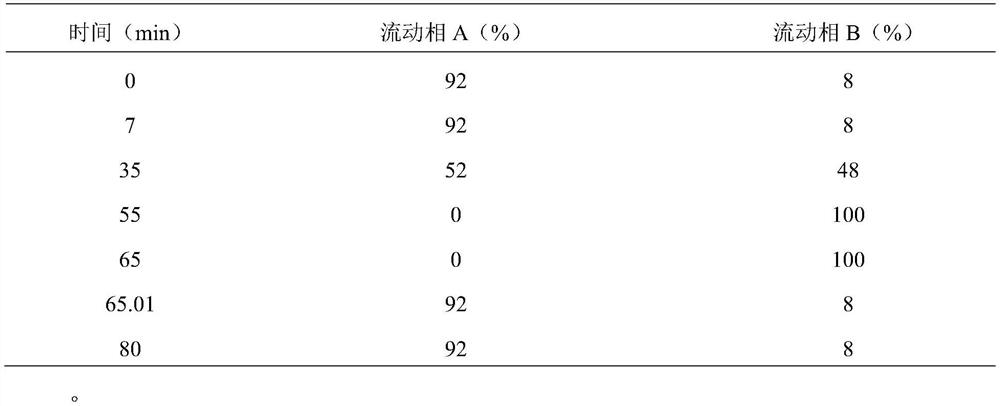
[0037] Liquid chromatographic analysis method for separation and determination of peramivir and its related substances (formula I)
[0038] Instrument: Agilent 1100 / 1260 High Performance Liquid Chromatograph
[0039] Column: Agilent Zorbax Bonus RP (250×4.6mm, 3.5μm)
[0040] Mobile phase: 10mmol / L dipotassium hydrogen phosphate solution (adjust the pH value to 4.5 with phosphoric acid) as mobile phase A, take mobile phase A-acetonitrile=40:60 as mobile phase B, and perform linear gradient elution as follows:
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com