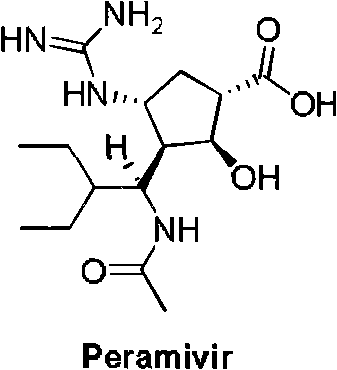
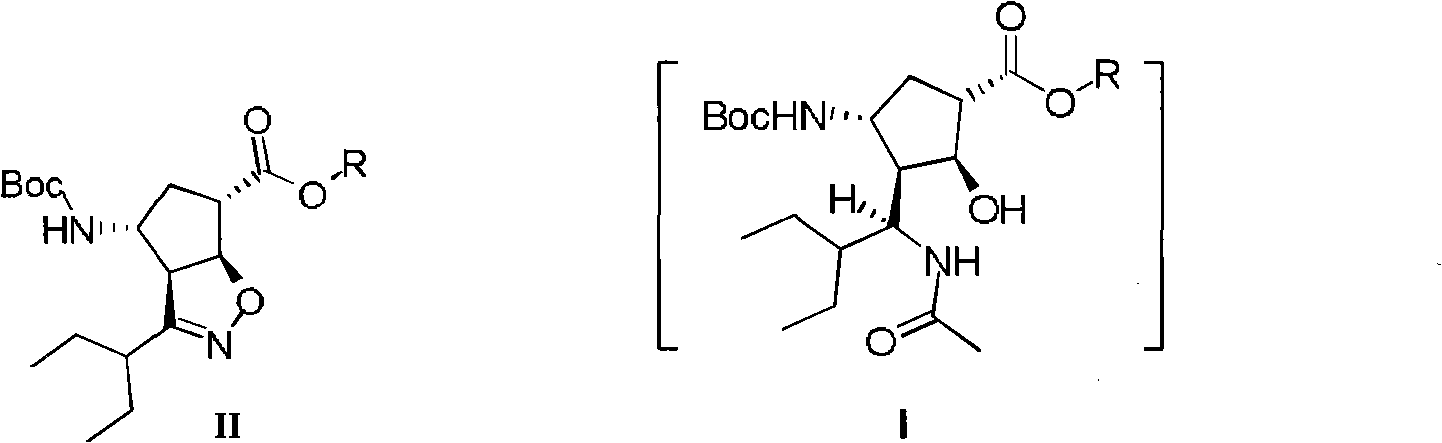
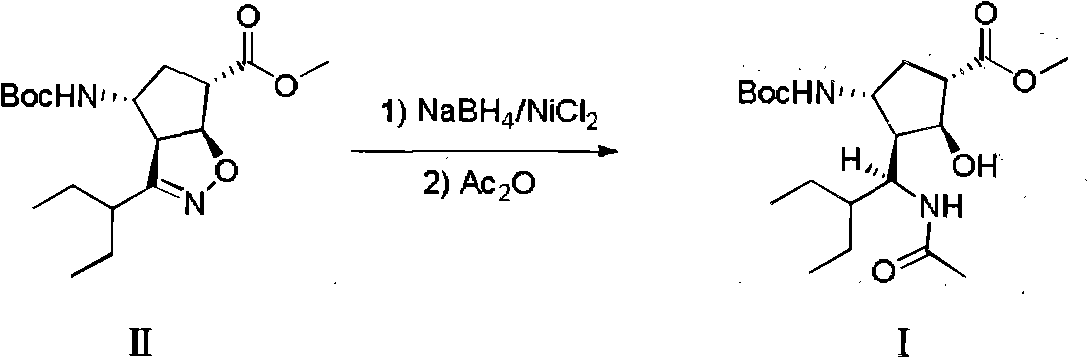New method for preparing peramivir key intermediate
A technology of intermediates and new methods, applied in the field of drug synthesis, can solve problems such as difficult mass production, complicated process, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1S, 2S, 3S, 4R, 1'S)-3-(1-acetylamino-2-ethylbutyl)-4-(Boc)-amino-2-hydroxycyclopentane-1-carboxylic acid methyl ester ( I) preparation:
[0023]
[0024] Compound II (1g, 2.82mmol), NiCl 2 ·6H 2 O (0.7g, 2.94mmol) was dissolved in a mixed solvent of 10mL methanol and 5mL tetrahydrofuran, cooled to -15°C, and NaBH 4 (0.3g, 7.9mmol) was slowly added to the above system, and the reaction was stirred after the addition (maintain the system temperature -5--10°C), and monitored by TLC (PE:EtOAC=5:1, color development in iodine cylinder). After the raw materials disappeared, acetic anhydride (3 g, 26 mol) was added dropwise to the reaction system, and stirring was continued at 0° C. for 2 hours (TLC:DCM:MeOH=10:1, the solution changed from black to green). Adjust the pH value to 9.6 with 25% ammonia water (2.6g, 0.038mol), spin off the methanol at low temperature, then add 20mL of water and 30mL of ethyl acetate to stir and separate the layers, and back-extract the aqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com