Novel method for producing peramivir trihydrate, and water-based drying thereof
A technology of peramivir trihydrate and carboxylic acid trihydrate, which is applied in the field of neuraminidase infection inhibitors and can solve the problems of difficulty in maintaining moisture value, natural drying method unsuitable for industrialized mass production, and GMP standards. , to achieve the effect of stable preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
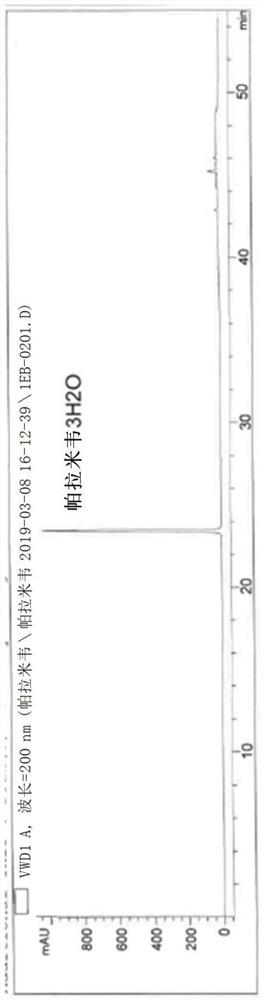
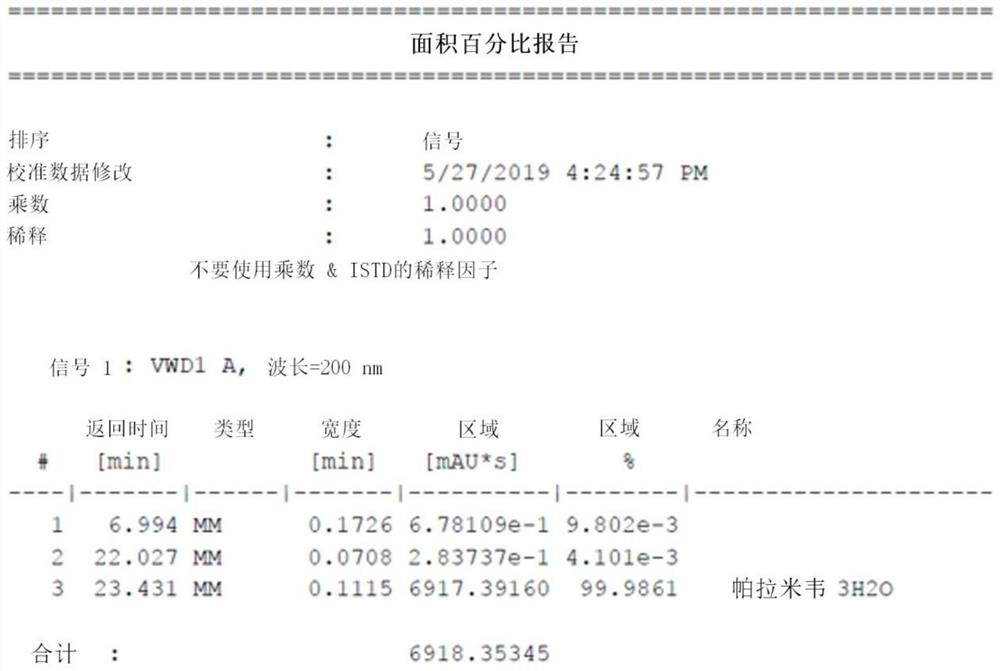
Image
Examples
Embodiment 1
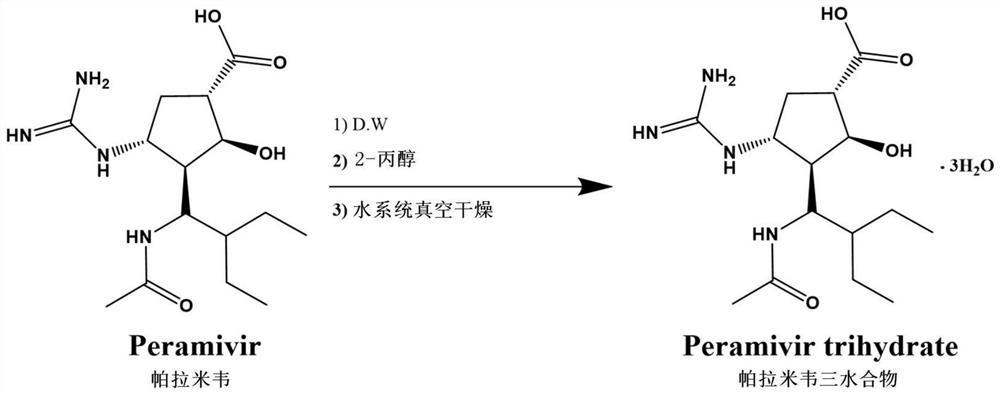
[0083] Example 1: Peramivir trihydrate ((1S, 2S, 3R, 4R)-3-[(S)-1-acetylamino-2-ethylbutyl]-4-guanidino-2-hydroxy Preparation of cyclopentane-1-carboxylic acid trihydrate) (1)
[0084]
[0085] Add 30.0kg of (1S, 2S, 3R, 4R)-3-[(S)-1-acetamido-2-ethylbutyl]-4-guanidino-2-hydroxyl ring in a 500.0L capacity reactor Pentane-1-carboxylic acid (Peramivir; Peramivir), and suspended in 240.0 L of purified water. The reactant was heated to about 95° C. to completely dissolve the suspension, stirred, and allowed to cool naturally. When the temperature of the reactant was 85°C, 45.0 L of 1-propanol (1-Propanol) was slowly added, and naturally cooled to 25°C while stirring. At this time, crystallites of peramivir trihydrate were produced at a temperature of about 40°C. When the temperature of the reactor reached 25° C. by natural cooling, it was stirred for about 12 hours to cause a phase transition of Form A. Then, the temperature of the reactor was cooled to 5° C., and stirred f...
Embodiment 2
[0087] Example 2: Peramivir trihydrate ((1S, 2S, 3R, 4R)-3-[(S)-1-acetylamino-2-ethylbutyl]-4-guanidino-2-hydroxy Preparation of cyclopentane-1-carboxylic acid trihydrate) (2)
[0088]
[0089] Add 30.0kg of (1S, 2S, 3R, 4R)-3-[(S)-1-acetamido-2-ethylbutyl]-4-guanidino-2-hydroxyl ring in a 500.0L capacity reactor Pentane-1-carboxylic acid (Peramivir; Peramivir), and suspended in 240.0 L of purified water. The reactant was heated to about 95° C. to completely dissolve the suspension, stirred, and allowed to cool naturally. When the temperature of the reactant was 85°C, 45.0L of 2-propanol (2-Propanol) was slowly added, and naturally cooled to 25°C while stirring. At this time, crystallites of peramivir trihydrate were produced at a temperature of about 40°C. When the temperature of the reactor reached 25° C. by natural cooling, it was stirred for about 12 hours to cause a phase transition of Form A. Then, the temperature of the reactor was cooled to 5° C., and stirred fo...
Embodiment 3
[0100] Example 3: Peramivir trihydrate ((1S, 2S, 3R, 4R)-3-[(S)-1-acetylamino-2-ethylbutyl]-4-guanidino-2-hydroxy Preparation of cyclopentane-1-carboxylic acid trihydrate) (3)
[0101]
[0102] Add 30.0kg of (1S, 2S, 3R, 4R)-3-[(S)-1-acetamido-2-ethylbutyl]-4-guanidino-2-hydroxyl ring in a 500.0L capacity reactor Pentane-1-carboxylic acid (Peramivir; Peramivir), and suspended in 240.0 L of purified water. The reactant was heated to about 95° C. to completely dissolve the suspension, stirred, and allowed to cool naturally. When the temperature of the reactant was 85°C, 45.0 L of 1-pentanol (1-Pentanol) was slowly added, and naturally cooled to 25°C while stirring. At this time, crystallites of peramivir trihydrate were produced at a temperature of about 40°C. When the temperature of the reactor reached 25° C. by natural cooling, it was stirred for about 12 hours to cause a phase transition of Form A. And, the temperature of the reactor was cooled to 5° C., further stirre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com