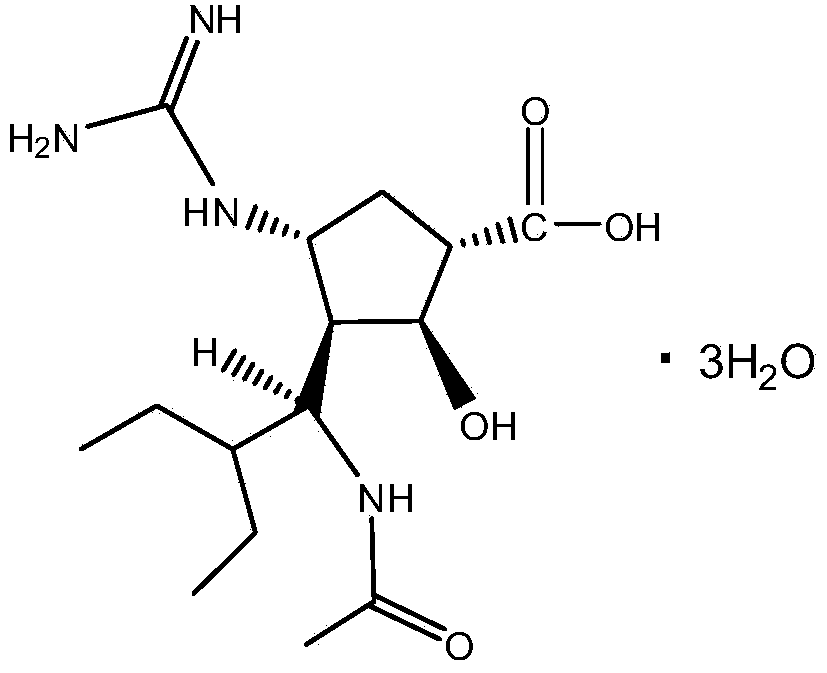
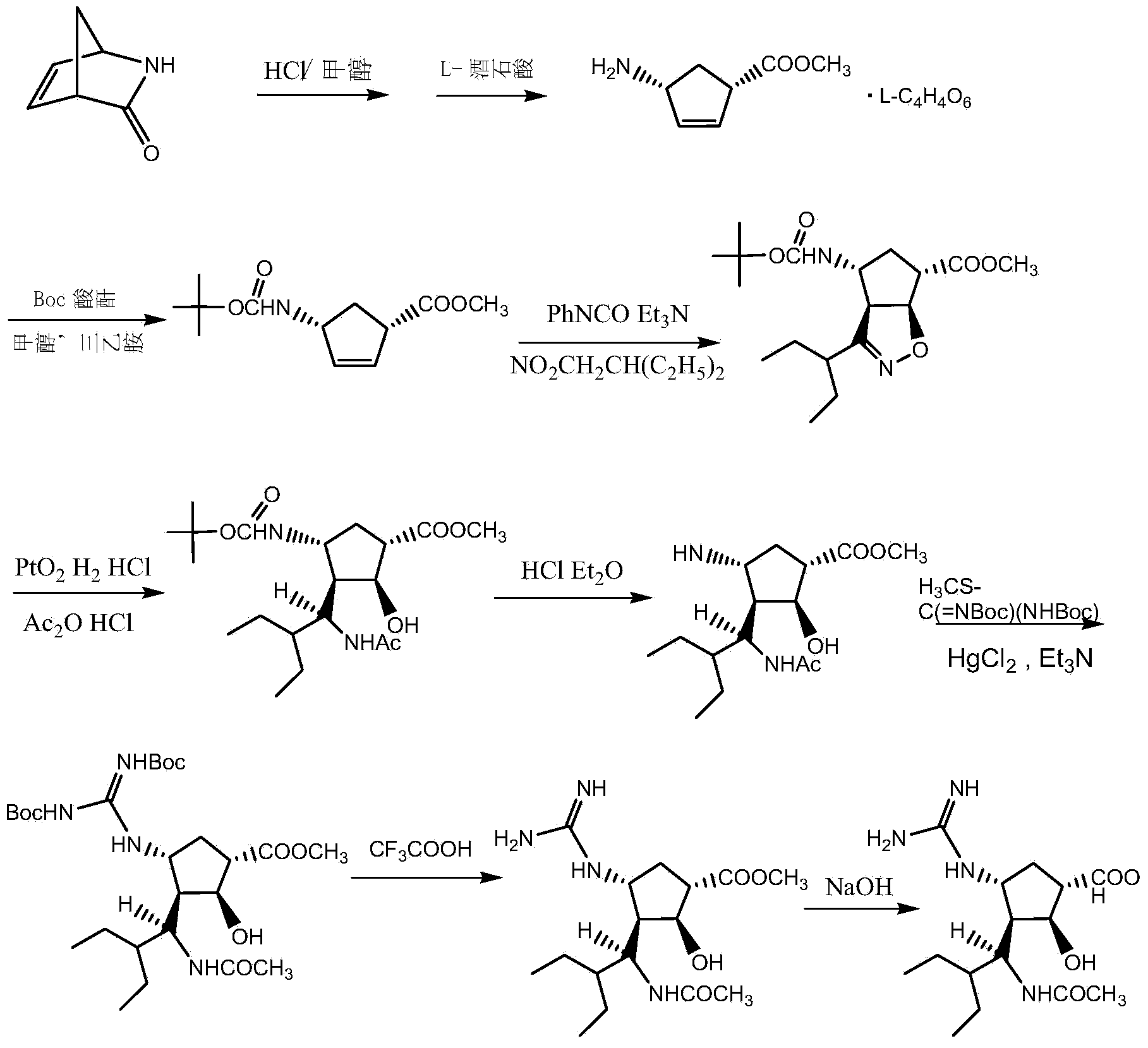
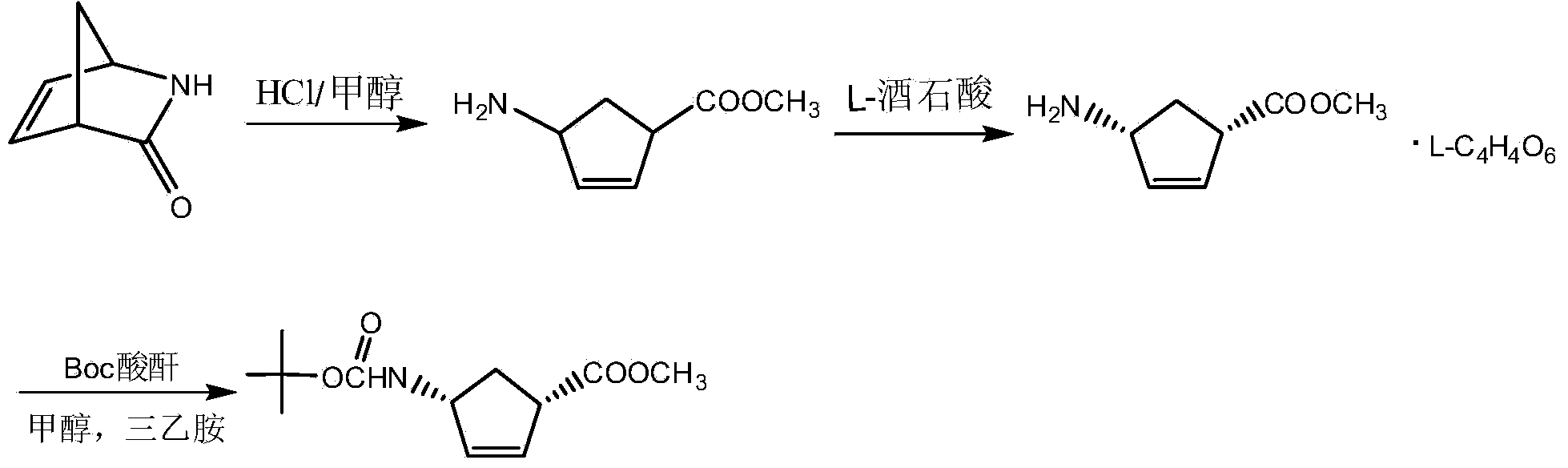
Method for preparing peramivir
A technology of amino and methyl carboxylate, which is applied in the direction of chemical instruments and methods, preparation of organic compounds, bulk chemical production, etc., can solve the problem of unsatisfactory purification treatment and affect the purity, yield and preparation of peramivir intermediate products Efficiency and other issues to achieve the effect of improving the preparation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The invention discloses a preparation method of peramivir, and those skilled in the art can refer to the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method of the present invention has been described through preferred embodiments, and relevant personnel can obviously make changes or appropriate changes and combinations to the preparation method described herein without departing from the content, spirit and scope of the present invention to realize and apply the present invention. Invent technology.
[0054] The 2-ethyl-N-hydroxybutyryl chloride described in the present invention can be prepared with reference to the preparation method in the patent CN1367776, and can also be prepared according to the method of the present inven...
Embodiment 1
[0059] Example 1: Preparation of PM01 ((1S,4R)-4-aminocyclopent-2-ene-1-carboxylic acid methyl ester L-tartrate)
[0060] 1. Preparation method
[0061] Put 1200g PM00 in a three-necked flask, add 3820mL of methanol, measure 785.2g of thionyl chloride, put it in a constant pressure dropping funnel, and add it dropwise to the above reaction system, control the reaction temperature at 15-30°C, and TLC after the addition is completed Detection, the reaction is complete. Methanol and acid gas were removed by rotary evaporation to give an oil. Dissolve the oil in 1920mL of methanol, control the temperature at 20-25°C, add 990.6g of L-tartaric acid and 720ml of water, add 734.6g of triethylamine dropwise, keep stirring for 0.5-1 hour after the addition is complete, and control the temperature at 20 -25°C, continue to stir for 1 hour, filter, wash the filter cake with 500ml of methanol, and dry in vacuum at 40°C for 5h to obtain 1368g of PM01. Yield 85.3%, ee ≥ 99% measured by HPL...
Embodiment 2
[0077] Example 2: PM02 ((1S,4R)-methyl-4-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopent-2-ene-1-carboxylic acid methyl ester) preparation
[0078] 1. Preparation method
[0079] Weigh 1300g PM01 and place it in a three-necked flask, add methanol 2135ml, Boc anhydride 1022g, then slowly add 1038g triethylamine dropwise, control the reaction temperature to less than 40°C, cool down to room temperature and stir for 1-2 hours after the dropwise addition is complete, TLC detection Until the reaction is complete, add 4550ml of water, add a small amount of PM02 seed crystals, stir out the solid, cool in an ice bath to 0-5°C and stir for 0.5 hours, add 2000ml of water, continue stirring for 2 hours, filter, wash the filter cake with 600ml of water, and then Washed twice with 1.5L petroleum ether, vacuum dried at 40°C for 5h to obtain 1045g PM02, yield 97.1%, HPLC measured ee≥99% (patent CN1358170 Example 3 report: yield 95.7%, purity unrecorded, by the present invention detection, i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com