Method for preparing peramivir key intermediate
An intermediate and key technology, applied in the field of drug synthesis, can solve problems such as complex process, low yield, and difficulty in mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
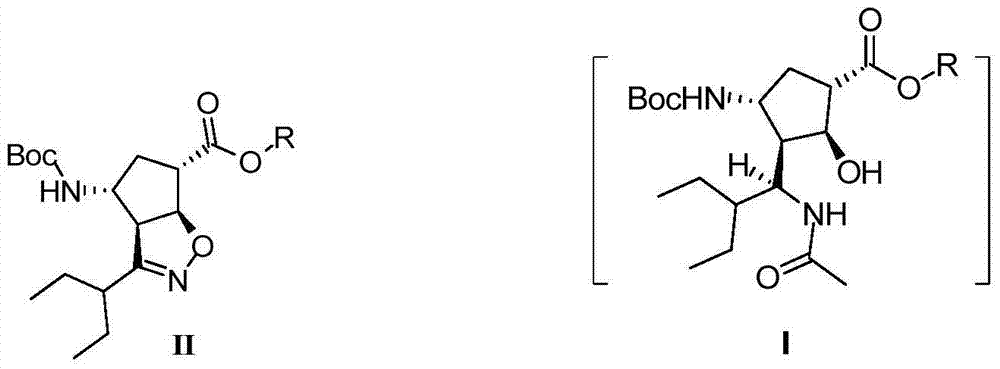
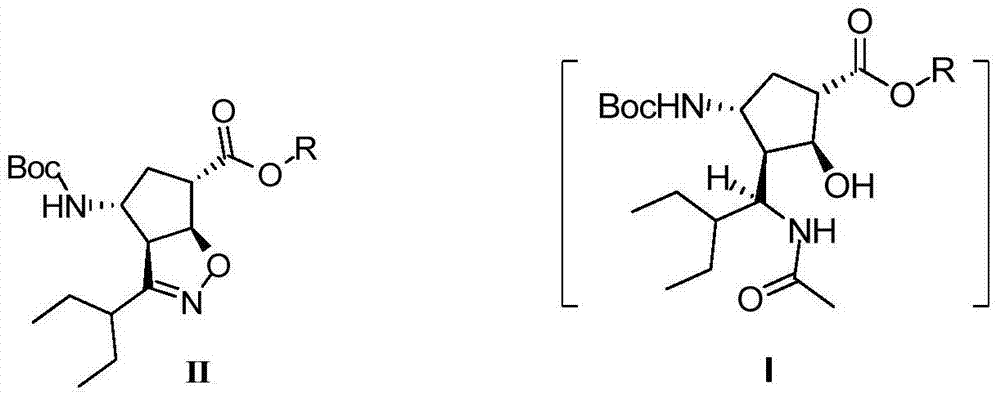
[0022] (1S,2S,3S,4R,1'S)-3-(1-Acetamido-2-ethylbutyl)-4-(Boc)-amino-2-hydroxycyclopentane-1-carboxylic acid methyl ester ( Ⅰ) Preparation:
[0023]
[0024] Compound Ⅱ (1g, 2.82mmol), NiCl 2 ·6H 2 O (0.7g, 2.94mmol) was dissolved in a mixed solvent of 10mL methanol and 5mL tetrahydrofuran, cooled to -15°C, and NaBH 4 (0.3g, 7.9mmol) was slowly added to the above system, and after the addition was completed, the reaction was stirred (maintaining the system temperature at -5–-10°C), and monitored by TLC (PE:EtOAC=5:1, color development in an iodine cylinder). After the raw materials disappeared, acetic anhydride (3 g, 26 mol) was added dropwise to the reaction system, and stirring was continued at 0°C for 2 hours (TLC:DCM:MeOH=10:1, the solution changed from black to green). Use 25% ammonia water (2.6g, 0.038mol) to adjust the pH value to 9.6, spin off methanol at low temperature, then add 20mL water and 30mL ethyl acetate to stir and separate, and the aqueous layer is bac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com