Peramivir dry powder inhalant and preparation method thereof
A dry powder inhaler and dry powder inhalation technology, applied in the field of medicine, can solve the problems of influence, influence on prescription, production and use, etc., and achieve the effects of reducing mortality, ensuring drug effectiveness, and excellent drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
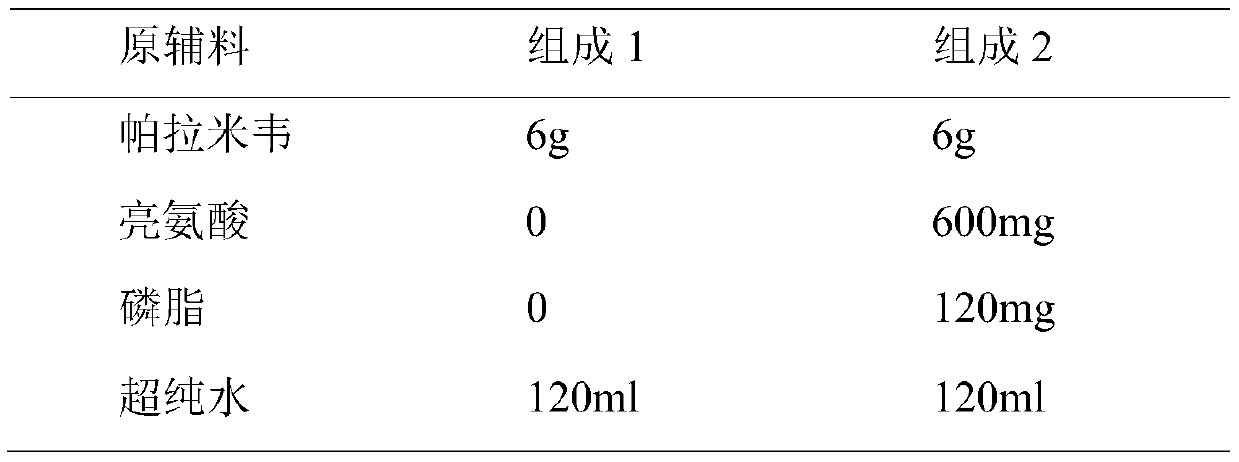
[0044] 1. The prescription of peramivir spray-dried powder is as follows
[0045] Table 1 Peramivir spray-dried prescription
[0046]
[0047] Process conditions: The spray drying process parameters are the inlet air temperature 200°C, the outlet air temperature 100°C, and the speed: 400ml / h. The obtained powder was passed through a 180-mesh sieve. The experimental results are as follows:
[0048] Table 2 Peramivir spray-dried powder experimental results (n=3)
[0049]
[0050] The experimental results showed that after adding phospholipids and amino acids, the density and particle size of the spray-dried powder were reduced, but the fluidity became worse.
[0051] 2. Mix dry powder of spray-dried powder and mannitol, fully mix the peramivir spray-dried powder of the above composition 1 and mannitol (w / w): 1:1; 1:2; 1:5. The experimental results are as follows:
[0052] Table 3 Peramivir spray powder mixed with mannitol experimental results (n=3)
[0053]
[005...
Embodiment 2
[0061] 1. Prescription 1: peramivir micropowder dry powder, the peramivir raw material drug is pulverized by airflow, and the dry powder is prepared under the conditions of feed speed 130V, feed pressure 0.45MPa, and pulverization pressure 0.45MPa.
[0062] 2. Prescription 2: Mix the dry powder prepared above with lactose Inhalac 120 to make a mixed powder, and mix at a mass ratio of 1:1.
[0063] 3. Prescription 3: Mix the dry powder prepared above with lactose Inhalac 400 to make a mixed powder, and mix at a mass ratio of 1:1.
[0064] Lung deposition rate test
[0065] The powders of the above-mentioned prescriptions 1-3 are respectively packed into capsules, 20 mg per capsule. Cover the NGI instrument, put it on the throat, seal it tightly with a sealing film, turn on the pump first, then turn on the flow meter, turn on the TPK instrument, then connect the flow meter to the throat, and use the pen test device to install the empty capsule and make a hole. Connect to a flo...
Embodiment 3
[0081] The peramivir trihydrate raw material of 1.3kg is pulverized with a jet mill, and at a feed rate of 130V, a feed pressure of 0.45MPa, and a pulverization pressure of 0.40MPa to obtain a dry powder, and then the dry powder is packed into hypromellose Vegetarian capsules, each capsule filled with 20mg dry powder. The dry powder particle size D10 is 1.934um; D50 is 4.415um; D90 is 8.858um. Angle of repose 34.8 degrees, bulk density 0.25g / cm 3 , tap density 0.41g / cm 3 , Carr flow index 59.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com