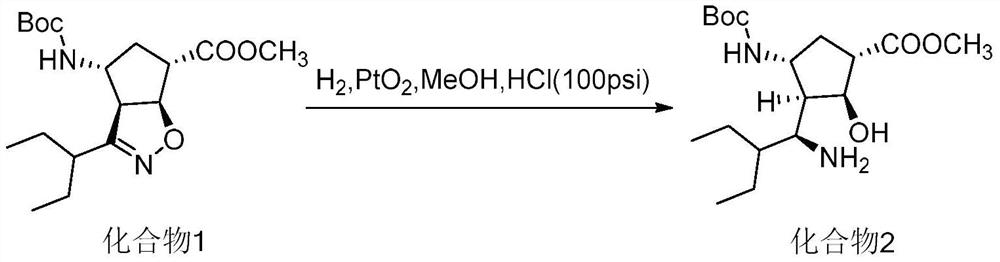
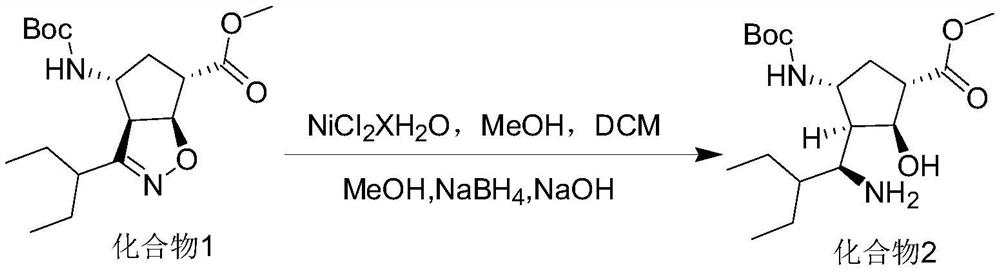Preparation method of peramivir intermediate
An intermediate and amino technology, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as long solid-liquid separation time, difficult material transfer, and increased processing costs, achieving significant social and economic benefits, reduced production costs, and side effects The effect of less product content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 (1.1eq nickel chloride hexahydrate)
[0024] Add 0.18g of sodium hydroxide, 6.41g of sodium borohydride, and 60mL of methanol into the beaker, and stir to dissolve.
[0025] Add 20.00 g of compound 1, 14.75 g of nickel chloride hexahydrate, 60 mL of dichloromethane, and 20 mL of methanol into the reaction flask, and cool down to -5°C. Keep the internal temperature at -5°C ~ 5°C and add the sodium borohydride and sodium hydroxide methanol solution prepared above dropwise, then react at -5°C ~ 5°C for 15 minutes, and stop the reaction when the compound disappears at 1 point as detected by TLC.
[0026] Add 19.79 g of ethylenediamine tetraacetic acid, 11.68 g of sodium nitrite, 20 mL of ammonia water, and 100 mL of water into a beaker, and stir to dissolve.
[0027] The above-mentioned prepared ethylenediaminetetraacetic acid, sodium nitrite, and aqueous ammonia solution were added to the reaction solution to quench the reaction, extracted with 200 mL of dich...
Embodiment 2
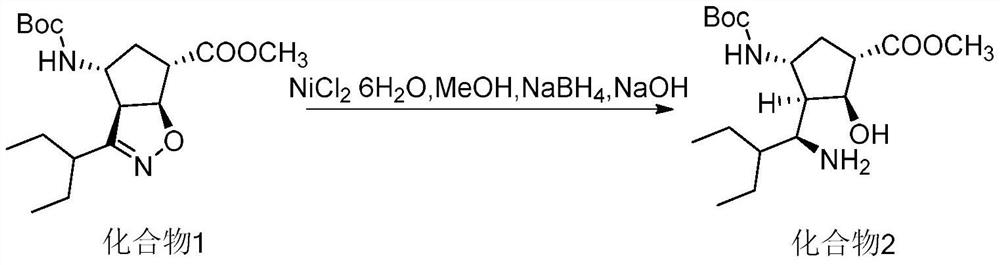
[0028] Embodiment 2 (0.05eq nickel chloride hexahydrate)
[0029] Add 0.17g of sodium hydroxide, 6.44g of sodium borohydride, and 60mL of methanol into the beaker, and stir to dissolve.
[0030] Add 20.00 g of compound 1, 0.67 g of nickel chloride hexahydrate, 60 mL of dichloromethane, and 20 mL of methanol into the reaction flask, and cool down to 20°C. Keep the internal temperature at -5°C ~ 5°C and add the sodium borohydride and sodium hydroxide methanol solution prepared above dropwise, then react at -5°C ~ 5°C for 15 minutes, and stop the reaction when the compound disappears at 1 point as detected by TLC.
[0031] Add 1.98g of ethylenediaminetetraacetic acid, 3.89g of sodium nitrite, 20mL of ammonia water, and 100mL of water into a beaker, and stir to dissolve.
[0032] The above-mentioned prepared ethylenediaminetetraacetic acid, sodium nitrite, and aqueous ammonia solution were added to the reaction solution to quench the reaction, extracted with 200 mL of dichloromet...
Embodiment 3
[0033] Embodiment 3 (0.2eq nickel chloride hexahydrate)
[0034] Add 0.17g of sodium hydroxide, 6.47g of sodium borohydride, and 60mL of methanol into the beaker, and stir to dissolve.
[0035] Add 20.00 g of compound 1, 2.68 g of nickel chloride hexahydrate, 60 mL of dichloromethane, and 20 mL of methanol into the reaction flask, and cool down to -5°C. Keep the internal temperature at -5°C-5°C and add the sodium borohydride and sodium hydroxide methanol solution prepared above dropwise, then react at -5°C-5°C for 15 minutes, and stop the reaction when the compound disappears at 1 point as detected by TLC.
[0036] Add 3.63g of ethylenediaminetetraacetic acid, 3.92g of sodium nitrite, 20mL of ammonia water, and 100mL of water into a beaker, and stir to dissolve.
[0037] The above-mentioned prepared ethylenediaminetetraacetic acid, sodium nitrite, and aqueous ammonia solution were added to the reaction solution to quench the reaction, extracted with 200 mL of dichloromethane,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com