Synthetic method of peramivir intermediate
A synthesis method and intermediate technology, applied in the field of pharmaceutical synthesis, can solve the problems of low yield, increase production cost, low total yield of peramivir finished product production, etc., and achieve lower production cost, simple processing, and improved reaction. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
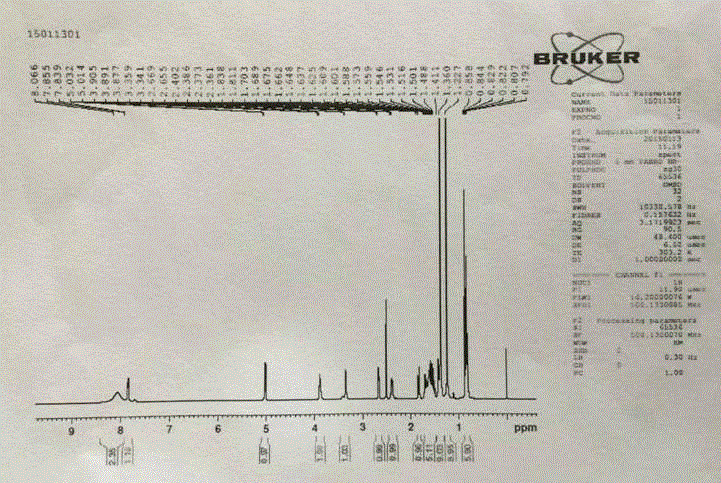
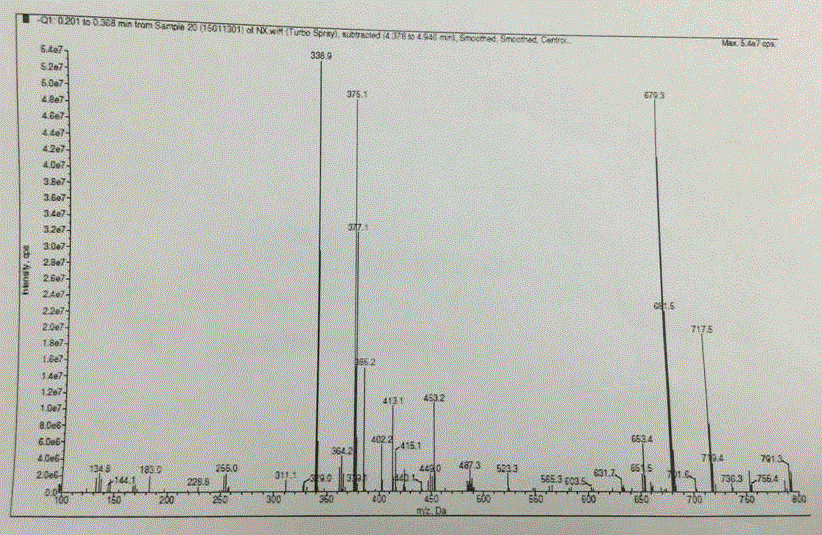
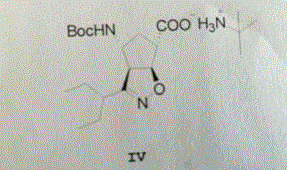
Image
Examples
Embodiment 1
[0029] Example 1 34.5 g (496 mmol) of hydroxylamine hydrochloride, 33.0 g of water, and 105.0 g of toluene were mixed, and 47.4 g (473 mmol) of 2-ethylbutyraldehyde was added with stirring. At 10 to 25°C, 79.4 g of a 30% sodium hydroxide solution (containing 23.8 g of sodium hydroxide) was added over 1 hour. After addition, continue to stir for 1.5h. Leave to stand for stratification, take the upper toluene layer, and use it directly for the next step. Will N - 63.2 g (473 mmol) of chlorosuccinimide (NCS) were suspended in 75 g of dimethylformamide, and cooled to 0-10°C. The toluene solution of the above oxime was added in 2.5 hours, and the reaction temperature did not exceed 25°C during the dropwise addition. After the addition was complete, stirring was continued for 1.5 hours. After completion of the reaction, 83 g of water was added, stirred for 30 minutes, and the reaction temperature was controlled not to exceed 30°C. The layers were allowed to stand, the aqueous...
Embodiment 2
[0030] Example 2 Will (1 S ,4 R )-(-)-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopent-2-ene-1-carboxylic acid methyl ester (compound I) 38.0g (157mmol), toluene 78g, 48g of triethylamine was mixed and heated to 50°C-55°C to dissolve. Then add PdCl 2 (PPh 3 ) 2 2.2 g (3.14 mmol), CuI 1.2 (6.28 mmol) g. To this solution, 120 g of a toluene solution containing 70.8 g (473 mmol) of 2-ethyl-N-hydroxybutyrimide chloride (compound II) was added dropwise. After the addition was complete, the reaction mixture was stirred at 60-65°C for about 9 hours. Add 80 g of water to the reaction solution to dissolve the solid, let stand to separate the toluene layer, and wash the toluene layer with water for 3 times. Then, the toluene layer was concentrated to remove 45 g of toluene. Add 63 g of 15% sodium hydroxide solution (containing 9.6 g of sodium hydroxide) to the concentrated toluene layer, and stir at room temperature for 10 hours. The toluene layer was washed with 15 g of water....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com