A novel process for the preparation of peramivir and intermediates thereof
A technology for a process and a compound is applied in the field of preparing peramivir or a pharmaceutically acceptable salt thereof, and can solve the problems of long synthesis route, low yield and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
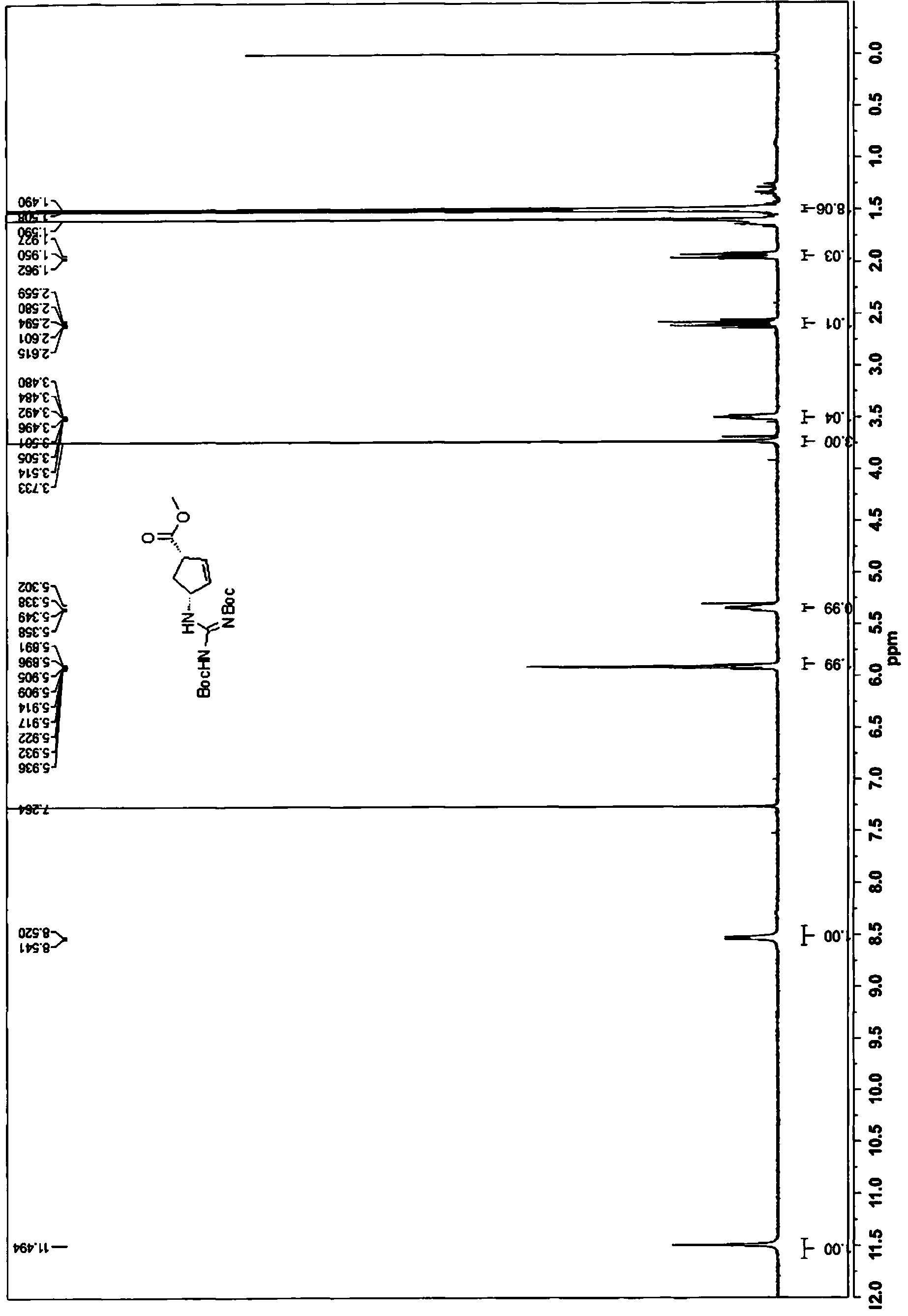
[0175] 1. (1S,4R)-methyl-4-(2,3-bis(tert-butoxycarbonyl)guanidino)cyclopent-2-ene-carboxylate (13)
[0176]
[0177] To a mixture of (1S,4R)-methyl-4-aminocyclopent-2-enecarboxylate tartrate 11 (7.29 g, 25 mmol) dissolved in dichloromethane (150 mL) at 0 °C was added ET 3 N (9 mL, 65 mmol) and the resulting mixture was stirred for 15 minutes. To this was added methyl tert-butyl(1H-pyrazol-1-yl)methylenedicarbamate 12 (7.38 g, 24 mmol). After the addition was complete, the completion of the reaction was monitored by TLC (PE:EtOAc=5:1). The organic phase was washed with water and brine, and anhydrous Na 2 SO 4 Dry overnight. The mixture was filtered and concentrated to give 13 as a white solid, which was used in the next step without purification.
[0178] MS(M+1): 384.
[0179] 1 H NMR (400MHz, CDCl 3 )δ11.49(s,1H),8.53(d,J=8.4Hz,1H),5.94-5.83(m,2H),5.38-5.31(m,1H),3.73(s,3H),2.60(dt , J=14.0, 8.5Hz, 1H), 1.94(dt, J=13.9, 4.7Hz, 1H), 1.50(d, J=7.4Hz, 18H) (see attac...
Embodiment 2
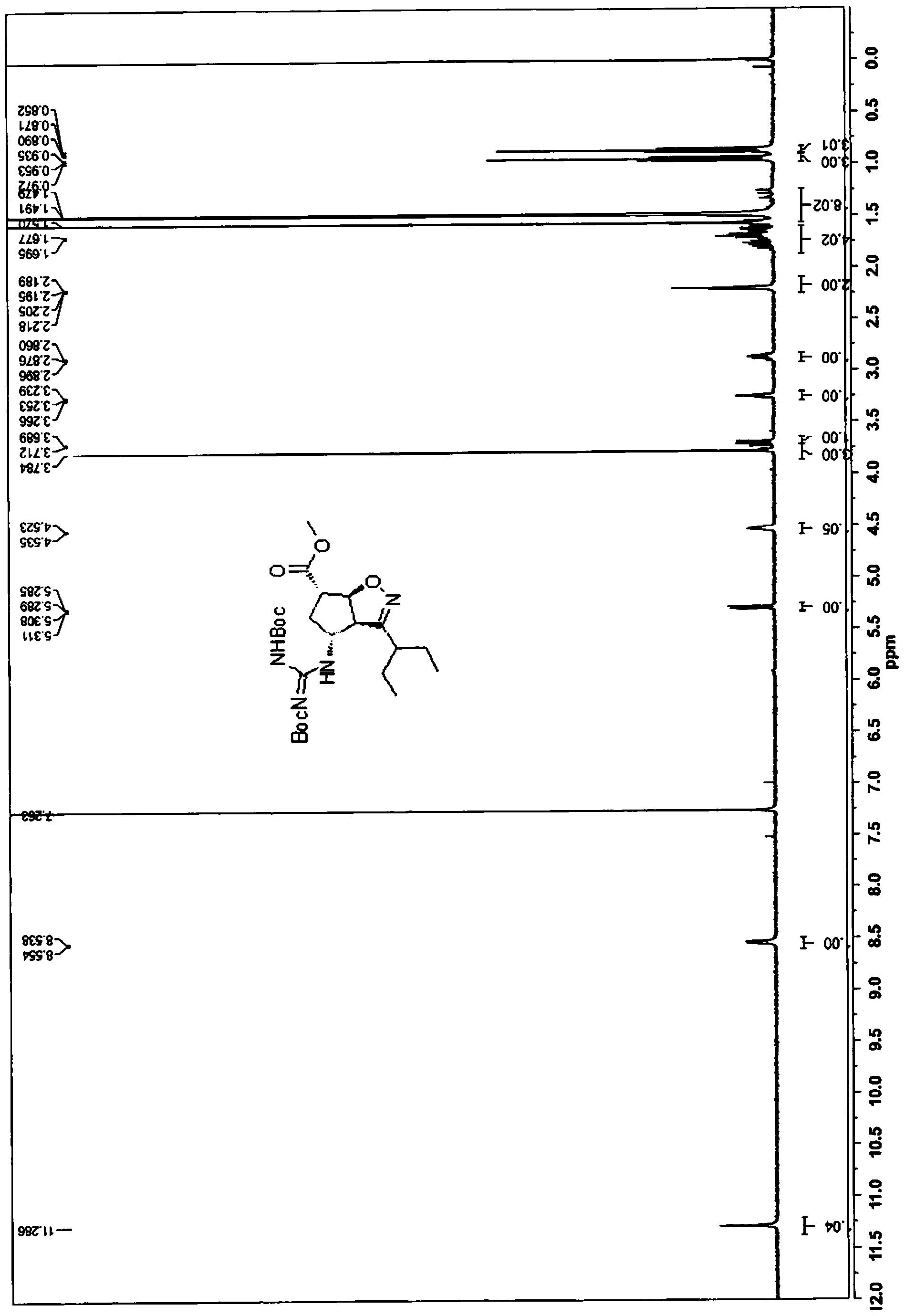
[0181] 2(3aR,4R,6S,6aS)-methyl-4-(2,3-bis(tert-butoxycarbonyl)guanidino)-3-(pentan-3-yl)-4,5,6,6α- Tetrahydro-3αH-cyclopentano[d]isoxazole-6-carboxylate (5)
[0182]
[0183] a) Preparation of 2-ethyl-N-hydroxybutyrimyl chloride (14)
[0184] Hydroxylamine hydrochloride (7.2 g, 0.1 mol) was dissolved in water (7 mL). Toluene (27 mL) was added followed by 2-ethylbutanal (10.0 g, 0.1 mol). The biphasic mixture was vigorously stirred while cooling. Sodium hydroxide solution (approximately 30%, 14.6 g, 0.11 mol) was added slowly (addition was strongly exothermic) to maintain the temperature between 15-25°C. The mixture was stirred for 60 minutes, after which the layers were left to stand. The organic phase was washed with water and brine, Na 2 SO 4 Dry overnight and use directly in the next step.
[0185] N-Chlorosuccinimide (NCS) (13.3 g, 0.1 mol) was suspended in dimethylformamide (DMF) (17 mL) and cooled to about 10 °C. The toluene solution prepared above (3.15 mol) ...
Embodiment 3
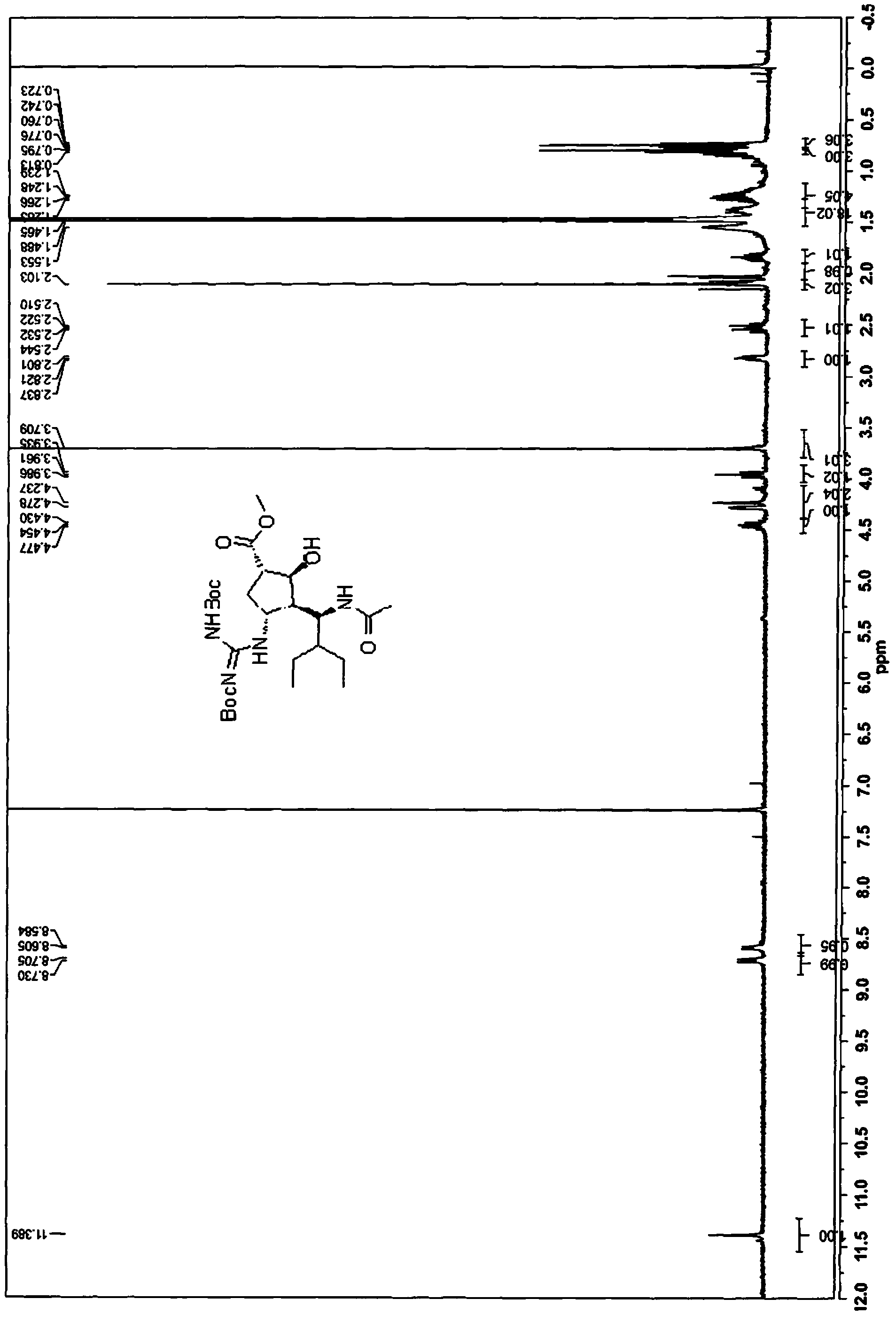
[0192] 3. (1S,2S,3S,4R,1'S)-methyl-3-(1-acetamido-2-ethylbutyl)-4-(2,3-bis(tert-butoxycarbonyl)-guanidino )-2-hydroxycyclopentanecarboxylate (16):
[0193]
[0194] Compound 15 (from Example 2, 5.0 g, 10.08 mmol) and nickel chloride hexahydrate (2.5 g, 10.5 mmol) were dissolved in methanol (40 mL). The green solution was cooled to -15°C while forming a suspension. Sodium borohydride (0.456 g, 12 mmol) was added to the reaction mixture at -5--10°C (the reaction was highly exothermic). A black suspension formed with gas evolution. After complete addition of sodium borohydride solution, the reaction mixture was stirred until TLC showed complete consumption of 15. A solution of acetic anhydride (15 g, 0.13 mol) was added slowly and maintaining the reaction temperature at 0-5 °C, the reaction mixture was stirred at 0 °C for 2-12 hours (the black solution turned to a green solution). The pH of the mixture was adjusted to approximately 9 by the addition of 25% aqueous ammonia....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com