Peramivir hydrate crystal, preparation method, medical compound and usage thereof
A technology of lamiver hydrate and hydrate, which is applied to the hydrate crystal of peramivir, the application field of medicine, and can solve the problems of difficulty in storage of anhydrous substances and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
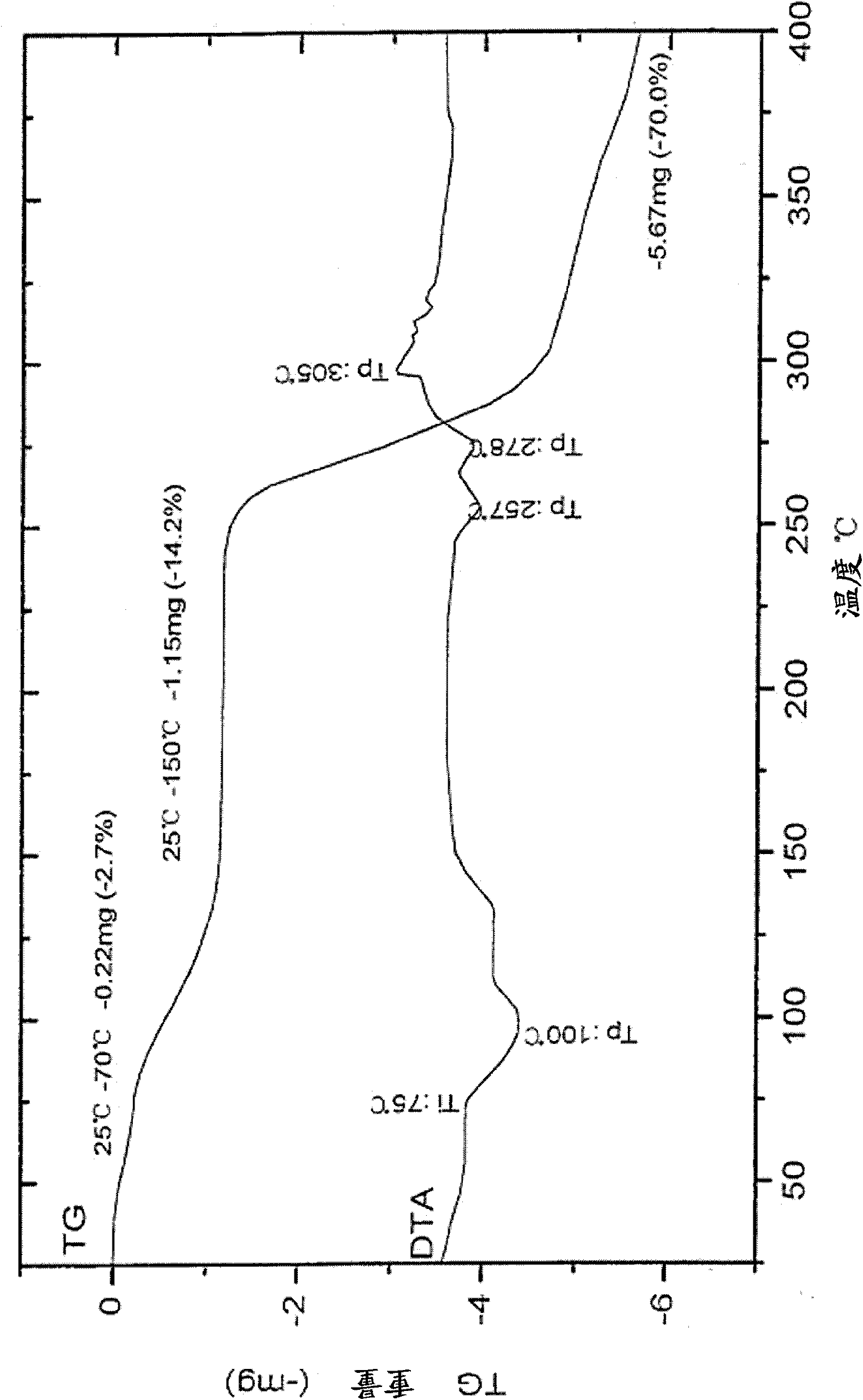
[0044] According to the peramivir hydrate crystal of the present invention, it contains 2.5 crystal waters. The stated amount of water of crystallization is a statistical value. Its preferred preparation method is as follows: (1) refining step: add peramivir crude product in methanol, heat to 80 ℃ for dissolving in distilled water, heat filtration, filtrate atmospheric pressure distillation, steam to boiling point 98 ℃, stop distillation, cool down to Stir at 25°C for 5 hours, filter, and wash with a small amount of water to obtain a white solid; (2) Vacuum drying step: put the white solid in a vacuum desiccator, add a desiccant to dry, vacuumize the system, and dry for more than 2 days to obtain paramilk Wei 2.5 Hydrate.
[0045] According to a further preferred embodiment of the present invention, the weight ratio of the peramivir crude product, methanol and distilled water is 1:2:4.5.
[0046]According to a more preferred embodiment of the present invention, the desiccant...
Embodiment 1
[0079] Embodiment 1, preparation of new crystals of peramivir 2.5 hydrate:
[0080] Add peramivir crude product in 5 liters of three-necked flasks (according to the method of publication number CN101538228A with (±) 2-azabicyclo [2.2.1] hept-5-en-3-one as starting material, implement according to CN101538228A Example 3, 9, 12, 14, 16) 600 grams, methanol (chemically pure reagent) 1500 milliliters, water 2700 milliliters. Heated to reflux temperature, stirred and dissolved, and filtered hot. The filtrate was distilled at normal pressure until the boiling point was 98°C, the distillation was stopped, the temperature was lowered to 25°C and stirred for 5 hours, filtered and washed with a small amount of water to obtain a white solid. Put the white solid in a vacuum desiccator, add anhydrous calcium chloride to dry, the system is evacuated (vacuum degree 0.08MPa), temperature is 25°C, and dried for more than 2 days. Obtain 579 grams of peramivir 2.5 hydrate crystals.
[0081]...
Embodiment 2
[0104] Embodiment 2, preparation of peramivir 2.5 hydrate new crystals:
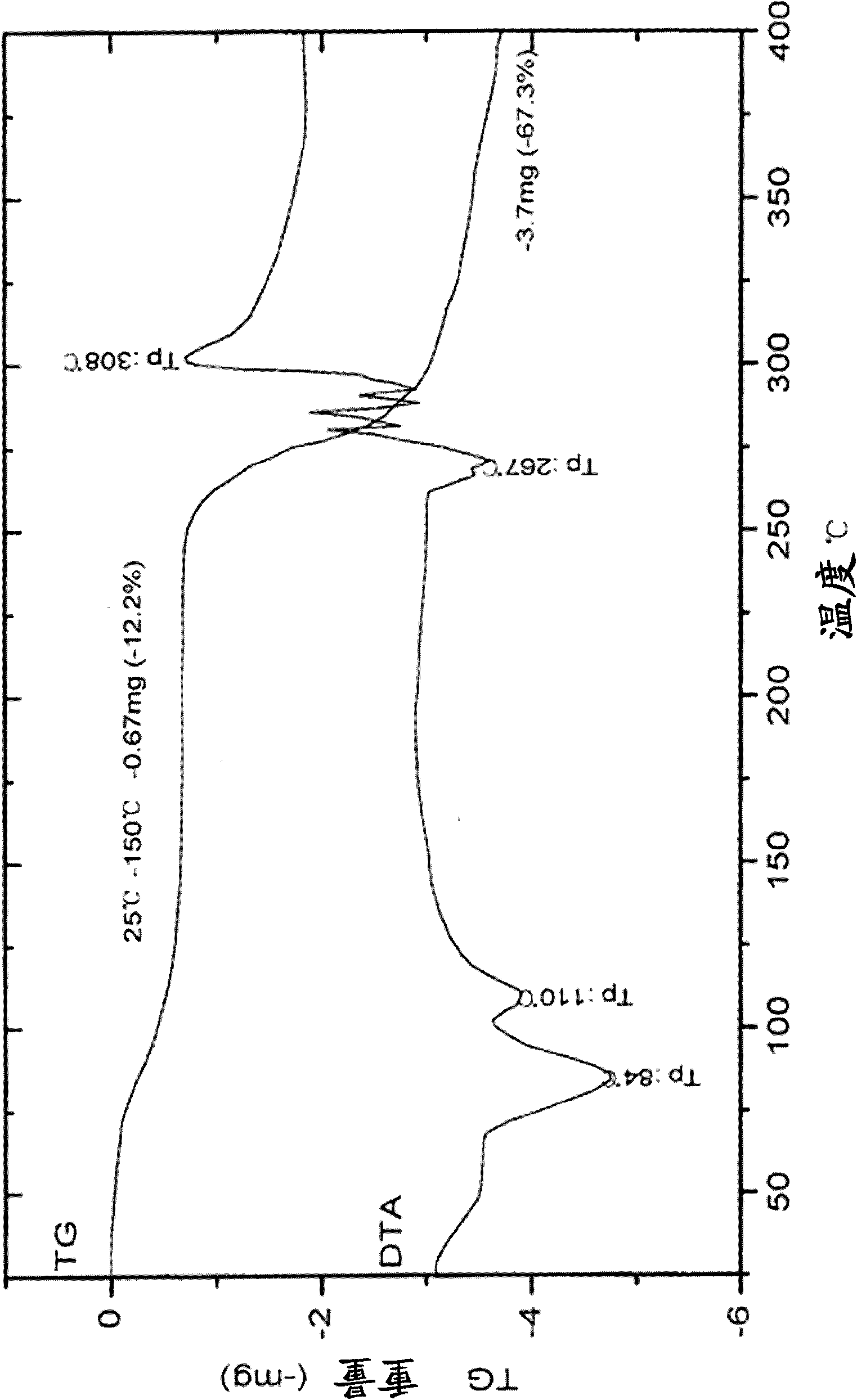
[0105]Add 200 grams of peramivir crude product, 485 milliliters of methanol, and 900 milliliters of water in a 2-liter three-neck flask. Heated to reflux temperature, stirred and dissolved, and filtered hot. The filtrate was distilled at normal pressure until the boiling point was 98°C, the distillation was stopped, the temperature was lowered to 25°C and stirred for 5 hours, filtered and washed with a small amount of water to obtain a white solid. Put the white solid in a vacuum desiccator and dry it without adding phosphorus pentoxide at a temperature of 30°C. The system is vacuumed (vacuum degree 0.09MPa) and dried for more than 2 days. 174 grams of peramivir 2.5 hydrate crystals were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com