Real-time fluorescence multiplex PCR primer probe for seven common respiratory system influenza virus pathogens and kit
A technology of influenza virus and primer probe, which is applied in the biological field to achieve the effect of saving time and preventing public health emergencies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
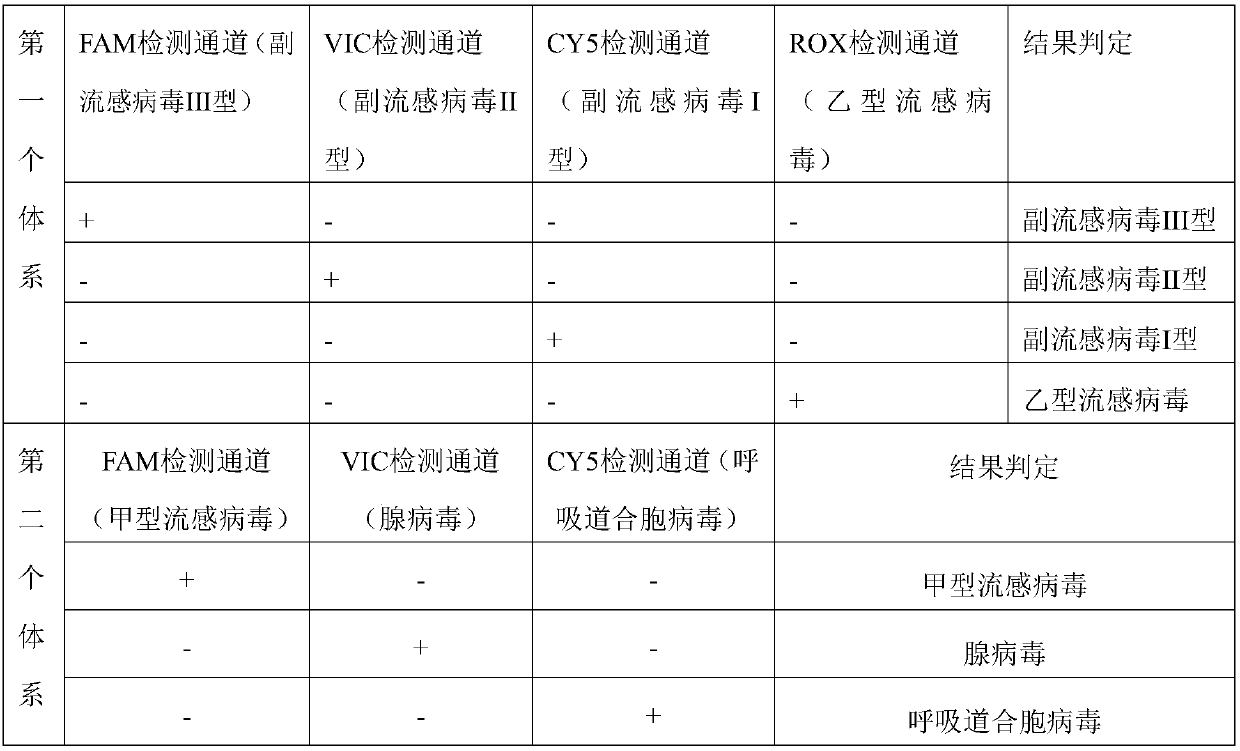
[0116] Formation of the first system:
[0117] Primer probes include:
[0118] The parainfluenza virus type I upstream primer of the nucleotide sequence shown in SEQ ID NO.1,
[0119] The parainfluenza virus type I downstream primer of the nucleotide sequence shown in SEQ ID NO.2,
[0120] A parainfluenza virus type I probe having the nucleotide sequence shown in SEQ ID NO.3, the 5' end of the parainfluenza virus type I probe is marked with a CY5 luminescent group, and the 3' end is marked with a fluorescent quencher Mission BHQ3;
[0121] The parainfluenza virus type Ⅱ upstream primer of the nucleotide sequence shown in SEQ ID NO.4,
[0122] Parainfluenza virus type Ⅱ downstream primer of the nucleotide sequence shown in SEQ ID NO.5,
[0123] A parainfluenza virus type II probe having the nucleotide sequence shown in SEQ ID NO.6, the 5' end of the parainfluenza virus type II probe is marked with a VIC luminescent group, and the 3' end is marked with a fluorescent quencher...
Embodiment 2
[0145] Operation and result judgment of embodiment 2 kit
[0146] 1. Genome extraction
[0147] After the throat swab is put into the sampling solution, it needs to be fully shaken and washed, and then left to stand at room temperature for 10 minutes. After the large lumps sink, the supernatant is taken and centrifuged immediately, and the subsequent precipitation can be used for nucleic acid extraction.
[0148] 2. Preparation of reaction system
[0149] The reaction system detected by the kit is 25 μL, and its configuration is as follows: 5 μL of 5× RT-PCR buffer (the buffer in tube B contains IAC template); 2.5 μL of 10× enzyme mixture; 2.5 μL of 10× primer-probe mixture ( The concentration of each primer is 0.5 μM, the concentration of each probe is 0.2 μM), RNA template 5 μL, and ultrapure water to make up to 25 μL.
[0150] 3. PCR reaction
[0151] Put the PCR tube into the fluorescence quantitative PCR instrument, and carry out the PCR reaction according to the follo...
Embodiment 3
[0157] The shelf life test of embodiment 3 kit
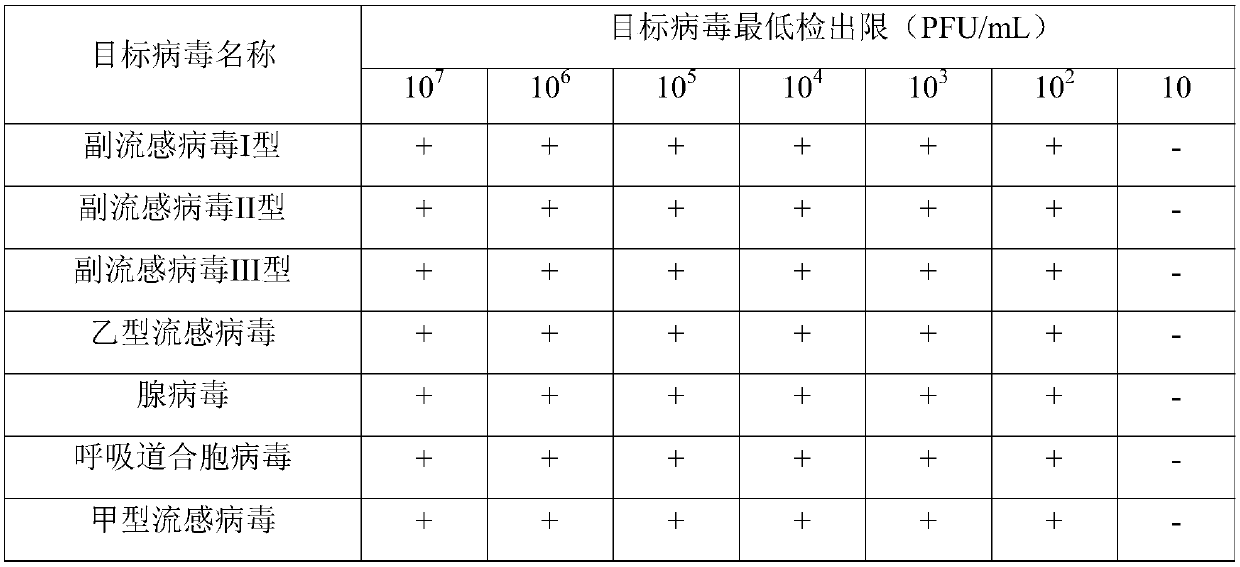
[0158] respectively by 10 5 PFU / mL The mixed template of parainfluenza virus type I, parainfluenza virus type II, parainfluenza virus type III and influenza B virus and the mixed template of adenovirus, respiratory syncytial virus and influenza A virus were used as test samples for evaluation, On day 0, aliquots were divided into 9 portions and stored in a -70°C refrigerator. The completed kits were stored at -20°C, and the kits of 0, 10, 15, 30, 60, 90, 120, 150, 180 and 200 days were used for storage test. The test results of the shelf life are shown in Table 2:
[0159] Table 2 shelf life test results
[0160] shelf life
[0161] It can be seen from Table 2 that the kit is stored in a -20°C refrigerator, and it is effective in detecting the seven target viruses at different storage periods. The experimental results show that the storage period of the kit is at least 6 months.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com