Nucleic acid composition, detection kit for influenza virus and micro-fluidic chip
A nucleic acid composition and microfluidic chip technology, applied in the biological field, can solve the problems of long period of virus isolation and culture and immunological detection, low PCR throughput and low detection sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0096] The preparation method of the above-mentioned microfluidic chip includes the step of preparing the PCR reaction reagent, the internal standard and the above-mentioned nucleic acid composition into freeze-dried powder.
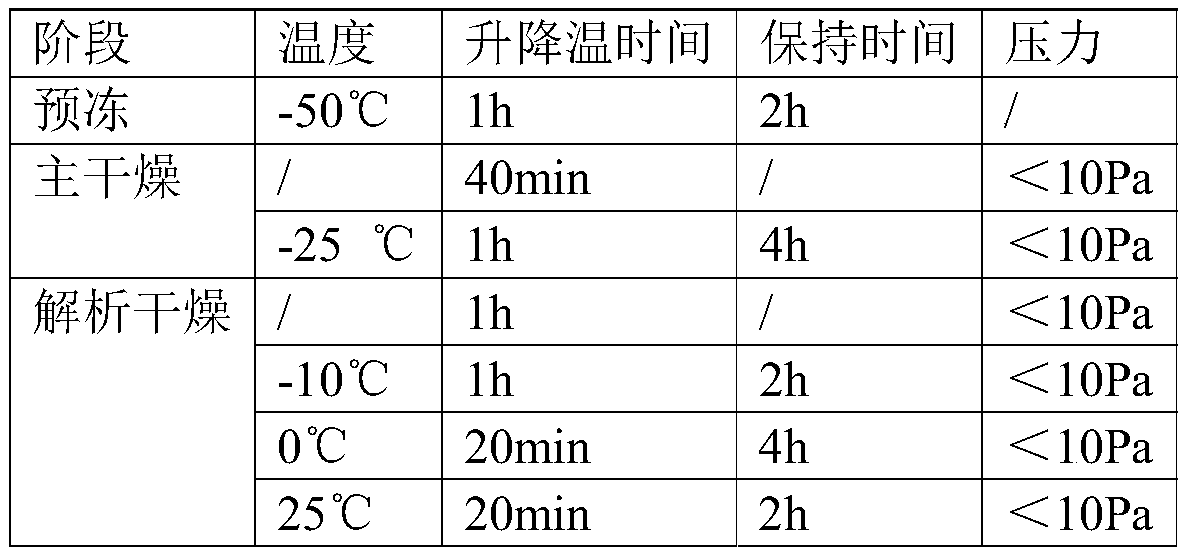
[0097] Specifically, the PCR reaction reagents, the internal standard and the above nucleic acid composition were respectively frozen and stored at -80°C for 8h-10h. Then carry out vacuum freeze-drying. Concrete vacuum freeze-drying process parameter is as follows table 1:
[0098] Table 1
[0099]
[0100]The PCR reaction reagent, the internal standard and the above nucleic acid composition after freeze-drying according to the above process can be stored and transported at room temperature, and the validity period of the reagent can be extended.
[0101] The above method of using the microfluidic chip includes step S110 and step S130. Specifically:
[0102] Step S110, extracting nucleic acid.
[0103] Specifically, the sample is added to the sam...
Embodiment 1
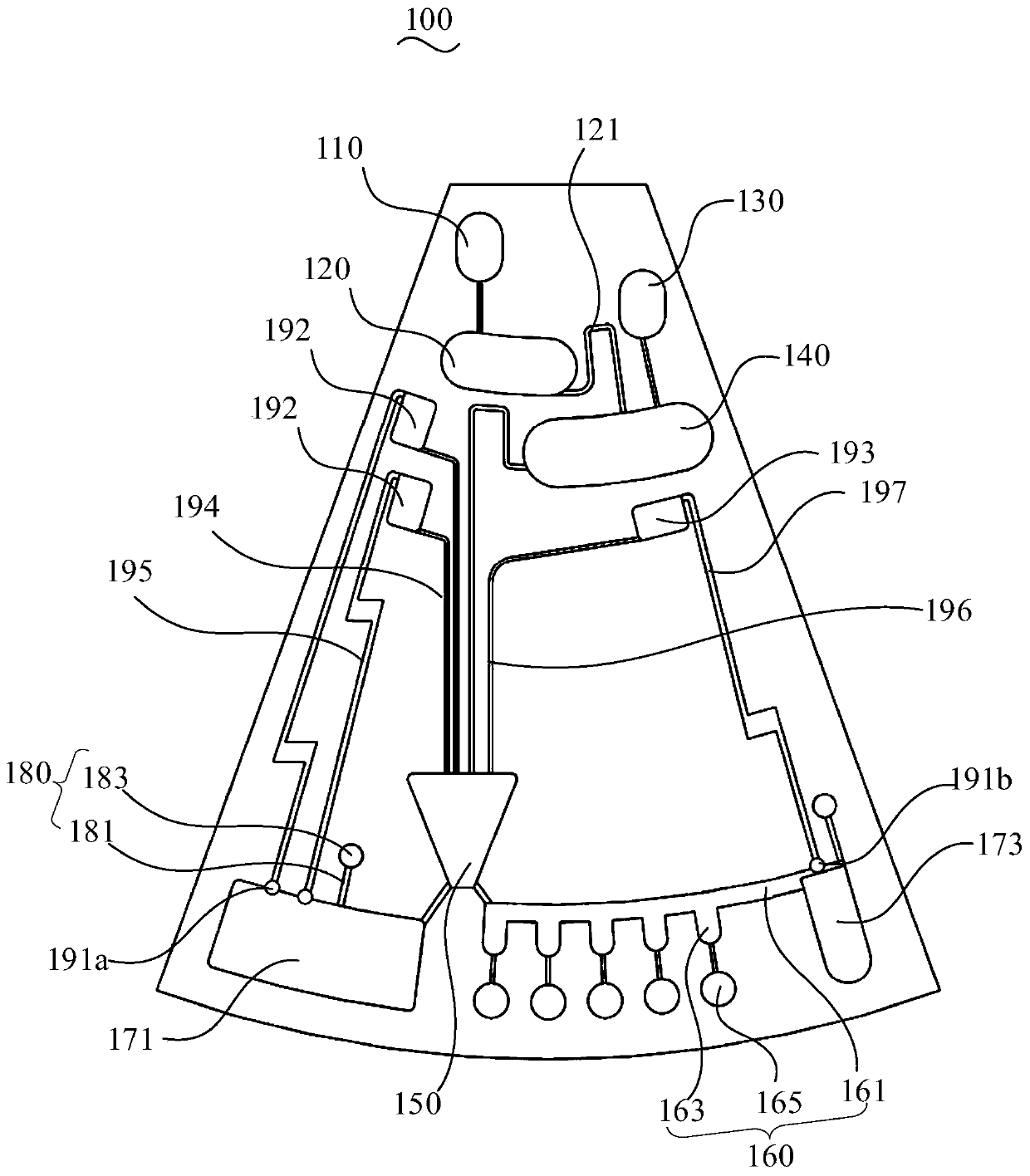
[0111] The structure of the microfluidic unit of the microfluidic chip of Example 1 is as follows figure 1 shown. The microfluidic unit includes a sampling pool, a buffer pool, a lysate storage pool, a lysis pool, a purification pool, a reaction pool, a waste liquid pool, and a liquid storage pool. A control valve is provided between the liquid storage pool and the waste liquid pool. The control valve is Paraffin valve. In the microfluidic unit:
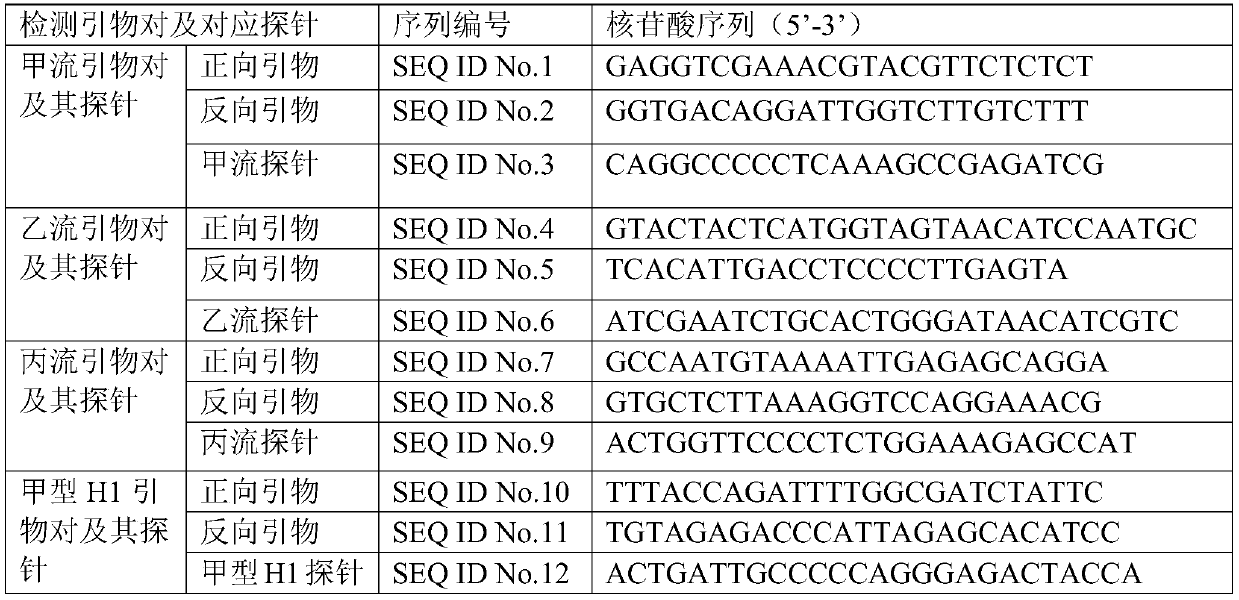
[0112] An internal standard is set in the buffer pool, and the internal standard is freeze-dried powder. The internal standard consists of an internal standard primer pair and an internal standard probe, and the specific sequences of the internal standard primer pair and internal standard probe are shown in Table 2;
[0113] Lysis solution is accommodated in the lysate storage tank, and the lysate is composed of guanidine hydrochloride with a final concentration of 5mol / L, C with a final volume percentage of 40%. 2 h 5 OH, Tris-...
Embodiment 2
[0122] (1) Obtain 20 clinical samples and number them from 1 to 20, including 10 influenza virus positive samples, 1 negative sample and 9 other subtype influenza A virus samples or virus samples that can cause influenza-like symptoms in humans. specifically:
[0123] Sample No. 1 is positive for influenza A (H10N2), sample No. 2 is positive for influenza B, sample No. 3 is positive for influenza C, sample No. 4 is positive for influenza A (H1N1), and sample No. 5 is positive for influenza A (H1N1). Influenza A H3N1 virus positive sample, sample No. 6 is a positive sample of influenza A H5N1 virus, sample No. 7 is a positive sample of influenza A H7N1 virus, sample No. 8 is a positive sample of highly pathogenic influenza A H7N9 virus, sample No. 9 Sample No. 10 is a positive sample of influenza A H9N1 virus, sample No. 10 is a positive sample of highly pathogenic influenza A H7N9 virus, sample No. 11 is a negative sample of influenza virus, sample No. 12 is a positive sample ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com