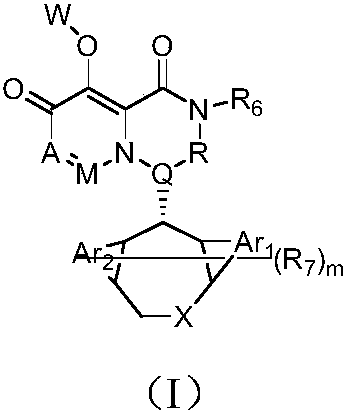
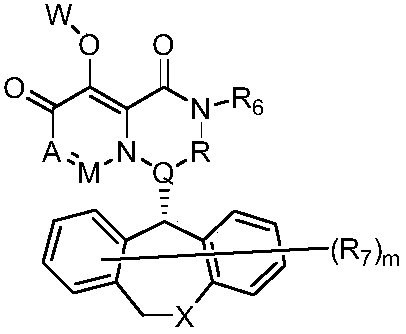
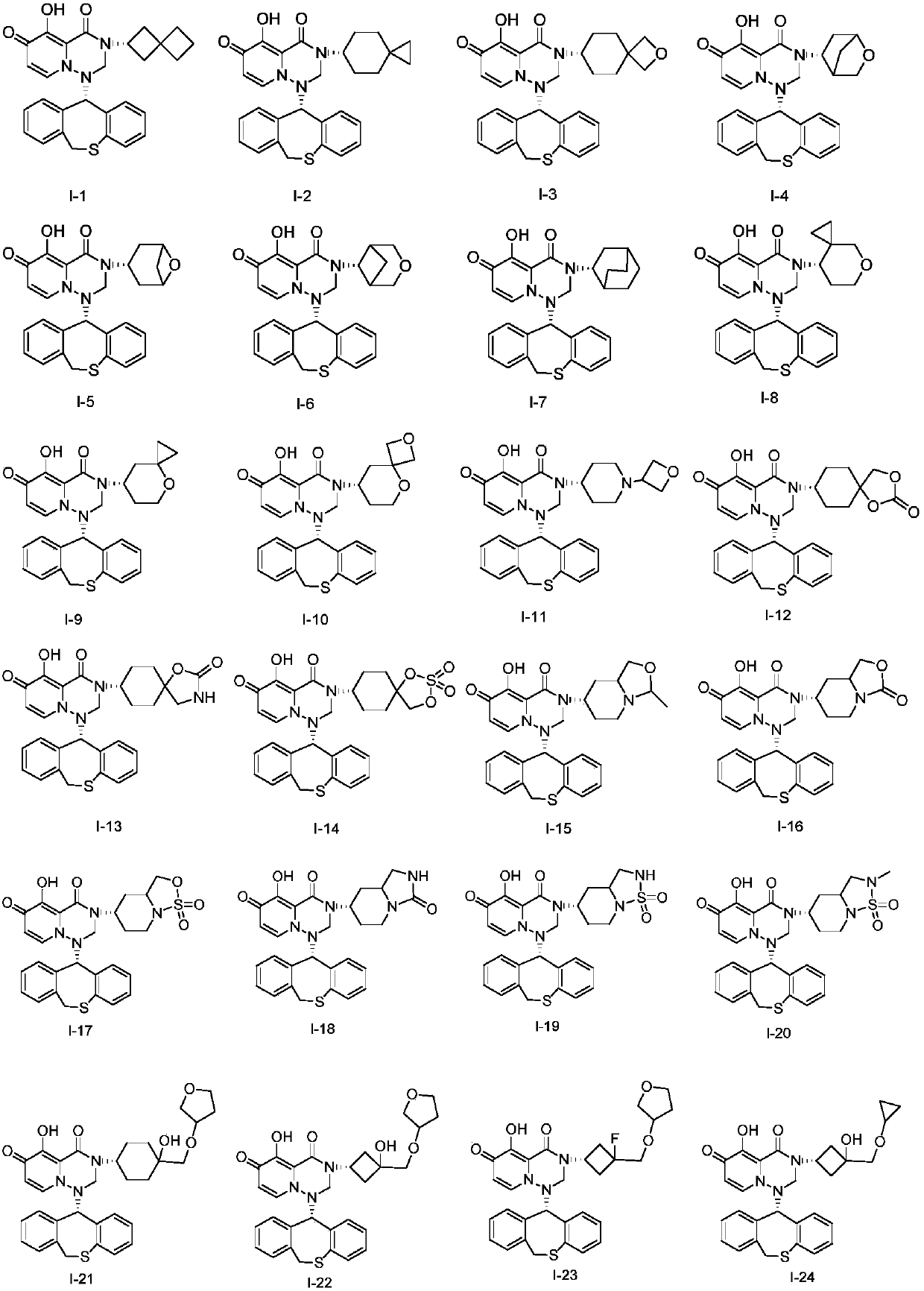
Pyridine derivative, composition thereof and application of pyridine derivative and composition as anti-influenza virus drug
A derivative, pyridone technology, applied in the direction of antiviral agents, active ingredients of silicon compounds, active ingredients of heterocyclic compounds, etc., can solve the problems of doubtful efficacy of neuraminidase inhibitors and ineffectiveness in severe patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1: Preparation (I-1)
[0104]
[0105] Preparation of Compound 1b: Compound 1a (2.0 g, 8.1 mmol), DBU (1.85 g, 12.2 mmol) and ethyl iodide (2.28 g, 14.6 mmol) were reacted in 20 mL of DMF at room temperature for 16 hours. Then add 100mL water to dilute and extract with EA. The organic phases were combined, washed successively with sodium thiosulfate, 0.5N HCl and saturated brine, dried over anhydrous sodium sulfate and spin-dried to obtain 2.1 g of an oily product.
[0106] The preparation of compound 1c: compound 1b (2.1g, 7.7mmol), Boc hydrazine (1.53g, 11.6mmol) and pyridine p-toluenesulfonate (5.78g, 23.1mmol) in N,N-dimethylacetamide (20mL ) at 60°C for 16 hours. After the reaction was completed, 100 mL of water was added to the reaction liquid, and then extracted with ethyl acetate (50 mL×3). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated. The crude product was separated by colu...
Embodiment 2
[0113] Embodiment 2: Preparation (I-26)
[0114]
[0115] Preparation of compound 26b: Compound 1d (360 mg, 1 mmol), 26a (116 mg, 1.2 mmol), TEA (303 mg, 3.0 mmol) and HATU (570 mg, 1.5 mmol) were stirred overnight in DCM at room temperature, then diluted with water, and extracted with DCM. The organic phases were combined, washed with saturated brine, dried and concentrated, and separated by column chromatography to obtain 320 mg of white solid. ESI-MS m / z 440.2(M+H) +
[0116] Preparation of compound 26c: Compound 26b (320 mg, 0.73 mmol) was dissolved in 4 mL of DCM, 1 mL of TFA was added and reacted at 0° C. for 6 hours. Spin dry, add 1N NaOH to adjust to basicity, and extract with DCM / iPrOH. The organic phases were combined, washed with saturated brine, dried and concentrated to obtain 195 mg of an oil, which was directly used in the next step.
[0117] Preparation of Compound 26d: Compound 26c (195 mg, 0.57 mmol) was dissolved in 5 mL of toluene, 30 mg of paraforma...
Embodiment 3
[0120] Embodiment 3: Preparation (I-27)
[0121]
[0122] Preparation of compound 27b: Compound 1d (360mg, 1mmol), 27a (136mg, 1.2mmol), TEA (303mg, 3.0mmol) and HATU (570mg, 1.5mmol) were stirred overnight in DCM at room temperature, then diluted with water, and extracted with DCM. The organic phases were combined, washed with saturated brine, dried and concentrated, and separated by column chromatography to obtain 345 mg of a yellow solid. ESI-MS m / z 456.2(M+H) +
[0123] Preparation of Compound 27c: Compound 27b (345 mg, 0.76 mmol) was dissolved in 4 mL of DCM, 1 mL of TFA was added and reacted at 0° C. for 6 hours. Spin dry, add 1N NaOH to adjust to basicity, and extract with DCM / iPrOH. The organic phases were combined, washed with saturated brine, dried and concentrated to obtain 170 mg of oil, which was directly used in the next step.
[0124] Preparation of Compound 27d: Compound 27c (170 mg, 0.48 mmol) was dissolved in 5 mL of toluene, 30 mg of paraformaldehyde ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com