Applications of novel antiviral protein C19orf66 in preparing medicines capable of resisting Zika virus
A Zika virus, anti-virus technology, applied in the field of protein engineering, to achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
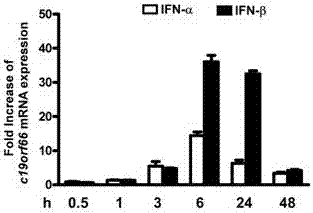
[0030] Example 1: Type I interferon IFN-α, IFN-β stimulates A549 cells, identification of endogenous C19orf66 expression level in cells
[0031] 1. Using A549 as a cell model, stimulate the cells with two type I interferons, IFN-α and IFN-β, at a concentration of 500U / ml.
[0032] 2. Collect cells at time points of 0.5h, 1h, 3h, 6h, 24h, and 48h, and extract total cellular RNA.
[0033] The specific operation of the Trizol method for total RNA extraction from adherent cells is as follows:
[0034] (1) RNase removal treatment: the glassware and consumables used for RNA extraction need to be soaked in 0.1% DEPC water overnight, and then sterilized by high temperature and high pressure steam twice for RNase inactivation treatment. Reagents required for RNA manipulation are prepared with high-pressure sterilized 0.1% DEPC water;
[0035] (2) After cell culture, the original culture medium was discarded, and the cells were washed twice with PBS;
[0036] (3) Add 1 ml TRlzol to t...
Embodiment 2
[0062] Example 2: Construction, identification and extraction of pMSCV-C19orf66 recombinant plasmid
[0063] 1. Primer design: Download the C19orf66 gene sequence from the GenBank database, and use PrimerPremier 5.0 software to design a pair of primers to amplify the full-length C19orf66 gene. The primers were synthesized by Shanghai Handsome Biotechnology Company. The primer sequences are as follows:
[0064] Forward: GGAAGATCTGCCATGTCTCAGGAAGGTGTGGAGCTGG
[0065] Reverse: CCGCTCGAGCTACTCCCTGGGCCCGCCCTC
[0066] 2. Obtain the target fragment: obtain the C19orf66 gene fragment by reverse transcription reaction, carry out PCR amplification with its cDNA, and electrophoresis all amplified products with 1.5% agarose gel (containing EB with a final concentration of 0.5 μg / ml) and Gel cutting recovery and purification.
[0067] 3. Extract pMSCV plasmid with QIAprep spin miniprep kit
[0068] 4. Double enzyme digestion reaction of pMSCV plasmid DNA and C19orf66 gene fragment PCR ...
Embodiment 3
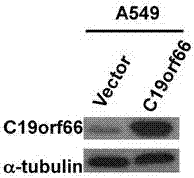
[0073] Example 3: Establishment and identification of a cell line stably and highly expressing exogenous C19orf66
[0074] 1. Preparation of retrovirus by calcium phosphate transfection
[0075] The recombinant plasmids pMSCV-puro, pMSCV-C19orf66 and packaging plasmid pIK were transiently co-transfected in 293T cells by calcium phosphate method.
[0076] The specific experimental steps are as follows:
[0077] (1) Take the 293T cell line growing in the logarithmic phase, digest and count the cells, inoculate in a 100mm culture dish at a density of 1×106 cells per well, add 10ml DMEM complete medium, place at 37°C, 5 %CO 2 Culture in a cell incubator (the number of cells prepared depends on the specific situation);
[0078] (2) After 24 hours, mix 20 μg of various recombinant plasmids, 20 μg packaging plasmid pIK, 380 μl 1×TE, 60 μl 2M CaCl2 in a 1.5ml centrifuge tube, and then slowly add 480 μl 2×HEPES drop by drop, at room temperature Stand still for 30 minutes;
[0079]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com