Antiviral protein and preparation method and application thereof
An anti-virus and protein technology, applied in the field of anti-viral proteins from fungi and its preparation, can solve the problems of unclear anti-viral components and unclear mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
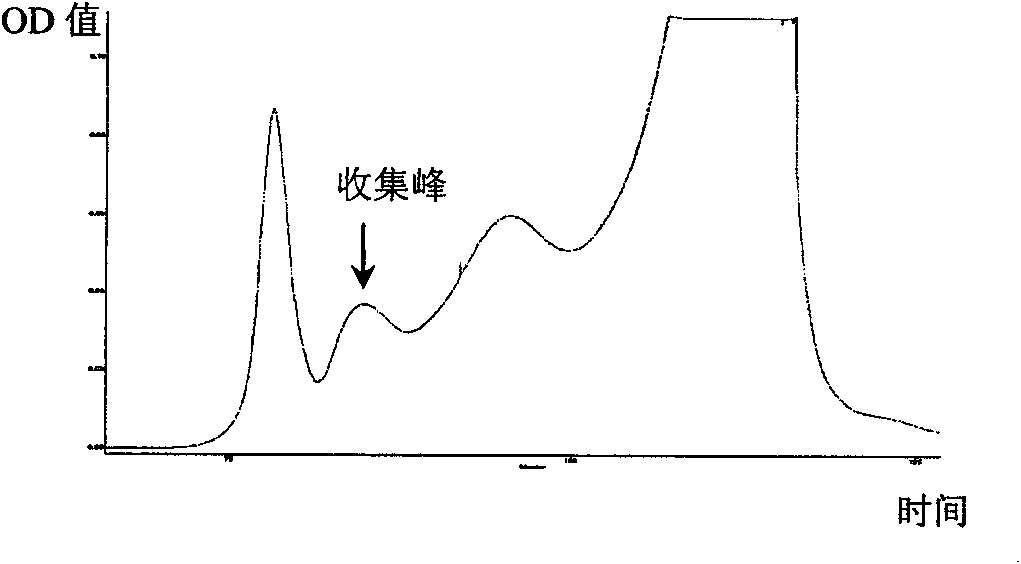
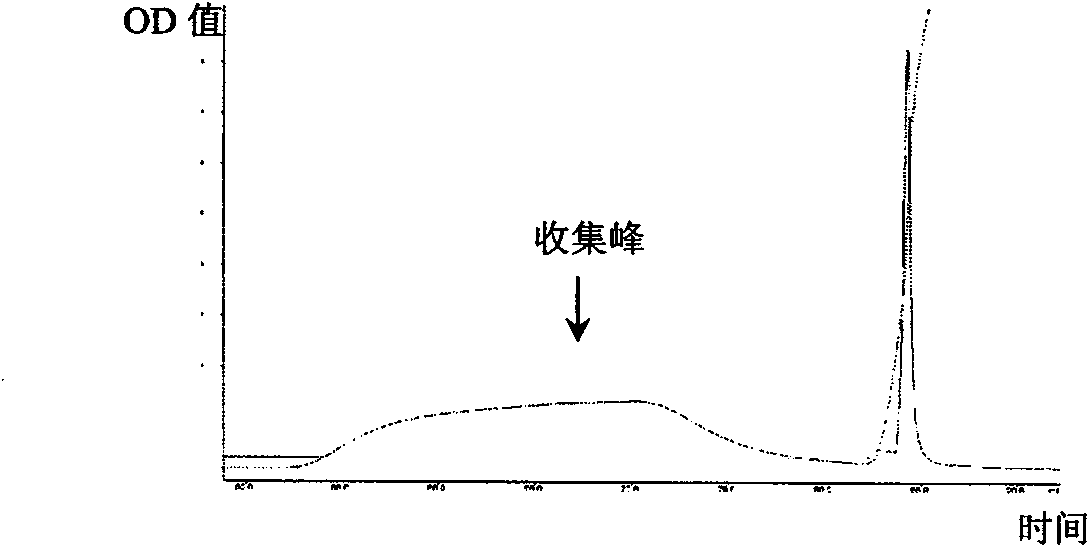
[0054] Example 1 Separation and purification of RC28 protein
[0055] (1) Coarse separation: the fruiting bodies of Umberella chinensis were collected from high-altitude mountain forests in Xiaojin County, Sichuan Province. After collection, they were immediately stored in ice packs and brought back.
[0056] Discard the handle of the fruiting body, take only the canopy, wash with distilled water, drain, add 5 times the volume of 4°C pre-cooled pH7.2, 0.02M PBS (containing 0.15M NaCl) solution and homogenate. Stir the homogenate mixture gently at 4°C overnight, centrifuge at 10,000g (20min) to remove the precipitate, and filter the supernatant with filter paper to remove the suspended matter. Use -20°C pre-cooled acetone to add the supernatant filtrate in a ratio of 2:1, mix immediately, After standing at -20°C for more than 2h, centrifuge at 10,000g (4°C) for 30min, discard the supernatant, dissolve the precipitate in 100ml, pH7.2, 0.02M (containing 0.3M NaCl) PBS solution, c...
Embodiment 2
[0060] Embodiment 2 Partial property experiment of antiviral protein RC28
[0061] (1) Stability: Treat the RC28 solution with boiling water at 100°C for 10 minutes, or use 0.25% trypsin to digest overnight at 37°C, then measure the activity against herpes virus type I, compared with the control group without any treatment, all There is more than 60% activity retention, indicating that RC28 has good thermal stability and enzymolysis resistance.
[0062] (2) When the pH value of the solution is less than 4, RC28 precipitates, but after returning to a neutral environment, the activity and solubility are well preserved; at the same time, from the perspective of ion exchange properties, the isoelectric point of RC28 is about 4.
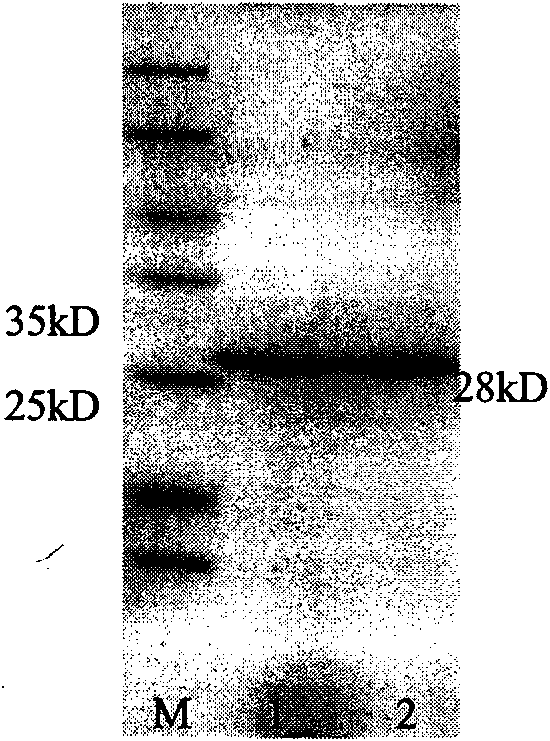
[0063] (3) The exact molecular weight of RC28 was determined to be 28.251kD by MALDI-TOF mass spectrometry.
[0064] (4) After SDS electrophoresis, RC28 was transferred to PVDF membrane, quickly stained with Coomassie brilliant blue R250, the band was ex...
Embodiment 3
[0070] Example 3 Determination of the complete sequence of RC28 protein
[0071] 1. Extract the total RNA of Rozites caperata
[0072] During the growing season of Umberella rugosa, the fresh tender tissues of the canopy were collected in the wild, stored in liquid nitrogen immediately and brought back, and the total RNA was extracted by the Trizol method, and identified by agarose gel electrophoresis, which confirmed that the obtained RNA was of good quality , no significant degradation occurred, and the electrophoresis results were as follows Figure 6 shown;
[0073] 2. Use the 3'-RACE method to amplify the cDNA of RC28
[0074] (1) Based on the obtained RC28 N-terminal sequence and referring to the codon preference of fungi Basidiomycetes, two sets of RACE degenerate primers were designed:
[0075] The outer primer is designed from the amino acid sequence GKLNMYNYA: GGNAAGYTBAACTGGTACAACTACGC
[0076] The inner primer is designed by the amino acid sequence YAVNEGFT: TA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com