Detection reagent for antiviral protein MxA and preparation method thereof
An anti-virus and protein technology, applied in the field of medical immunodiagnosis, can solve the problems affecting the test precision and operation error, and achieve the effect of shortening the test time, simplifying the operation procedure, and good applicability of the reagents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Preparation of latex reagent
[0030] There are differences between preparation methods 1 and 2 in the combination of surfactants.
[0031] Preparation method 1: Prepare activated latex particles: Take 0.18mL of carboxylated polystyrene microspheres with a particle size of 333nm and a concentration of 10mg / mL, add 3.6mL of 25mM MES with pH6.5, stir for 10min, and add 7.04mg of EDC activator powder After stirring at room temperature for 2.5 hours, the precipitate was collected by centrifugation at 12000 rpm, and the precipitate was resuspended in 1.8 mL of 25 mM MES with pH 6.5.
[0032] Preparation of sensitized latex particles: 0.18 mL of MxA polyclonal antibody with a concentration of 4.0 mg / mL was added to 1.8 mL of 25 mM MES at pH 6.5, and stirred for 1 min to prepare a solution of the antibody to be coupled. Quickly pour the activated latex particles in (1) into the antibody solution to be coupled and stir for 2.5 hours. The precipitate was collected by centri...
Embodiment 2
[0042] Reagent sensitivity (blank limit)
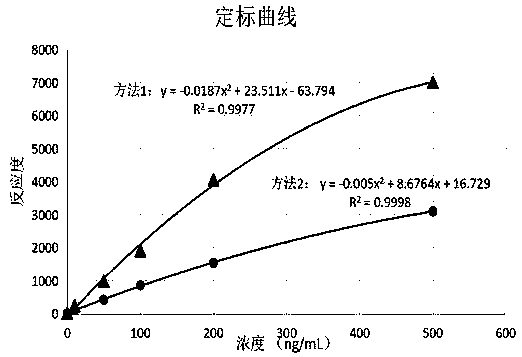
[0043] Using the reagents in Example 1, select the zero-value calibrator as a blank sample test, and test the concentration on the POCT device. The test was repeated 20 times, and the mean (X) and standard deviation (SD) of the 20 test results were calculated according to the standard curve established in Example 1, and X+2SD was calculated as the sensitivity of the reagent. The test results are shown in Table 1, showing that the sensitivity of the reagent obtained by Preparation Method 1 is 5 ng / mL, and the sensitivity of the reagent obtained by Preparation Method 2 is 15 ng / mL, indicating that the combination of surfactants can significantly improve the sensitivity of reagent detection . The clinical reference value for the detection of the antiviral protein MxA (MxA) is about 50ng / mL, and the sensitivity of the MxA latex-enhanced immune turbidimetric reagent prepared in Preparation Method 1 of the present invention is 10 times sma...
Embodiment 3
[0047] Reagent repeatability
[0048] With the single reagent test prepared in Example 1, the MxA concentration is the control substance of the normal value level of about 50ng / mL and the control substance of the abnormal value level of the MxA concentration of about 200ng / mL, each repeated test 10 times, calculate the measured value Mean (X) and standard deviation (SD). Calculate the coefficient of variation (CV) according to formula (1). The test results are shown in Table 2. According to the measurement results, the coefficients of variation CV=5.35% and 4.53% were calculated, which met the technical requirements of reagent CV<10%.
[0049] CV = (SD / X) × 100% ………………………(1)
[0050] In the formula:
[0051] CV - coefficient of variation;
[0052] SD - standard deviation;
[0053] X - the average value of the measured values.
[0054] Table 2. Repeatability measurement results of Example 1
[0055] Example 1 repeatability repeatability Assay number ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com