Method for preparing recombined blue algae antiviral protein and application thereof
An anti-virus and protein technology, applied in the field of genetic engineering, can solve problems such as inactivity, complicated purification methods, and lack of amino acids in fusion expression, and achieve the effect of simplifying the purification route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Construction and sequence analysis of plasmid pET3c-SUMO-CVN
[0038] 1. Design of primer sequences
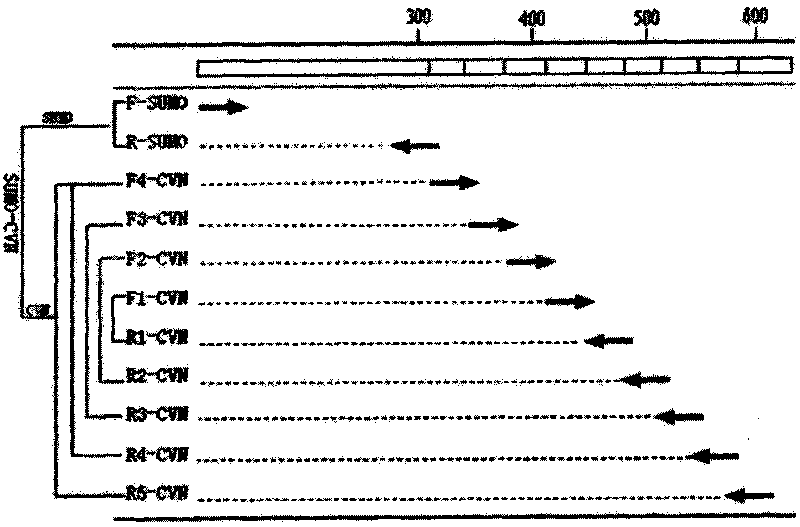
[0039] According to the codon preference of Escherichia coli, the original sequence of CVN was optimized, eleven primers were synthesized, and the complete sequence of SUMO-CVN was synthesized by multiple PCRs. The synthesis strategy was as follows: figure 1 shown.
[0040] The designed primers were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd., and the sequences are shown in Table 1.
[0041] Table 1 Primers used to synthesize the full-length sequence of SUMO-CVN
[0042]
[0043] 2. Synthesize the full sequence of SUMO-CVN by PCR
[0044] The synthesis was carried out in two steps. First, the CVN gene was synthesized by multiple PCRs, and the first PCR primers were F1-CVN and R1-CVN. The reaction system is 1 μl of each primer, 8 μl sterile ddH2O, 10 μl Taq PCR MasterMix, the reaction mixture is denatured at 94°C for 1 min,...
Embodiment 2
[0047] Expression, purification and identification of embodiment 2 recombinant protein
[0048] 1. Screening and induction of expression strains
[0049] Pick the single clone that has been sequenced correctly, extract the plasmid and transform it into the expression host BL21(DE3), use a plate containing ampicillin to screen out positive transformants, pick the single clone and put it in 3ml of LB medium containing ampicillin, at 37°C, Cultivate to OD at 180rpm 600 = 0.6 to 1.0. Take 1ml as uninduced control, add IPTG to the rest to a final concentration of 1mmol / L, induce at 37°C for 4 hours, collect the bacteria by centrifugation, and resuspend the bacteria pellet in 100μl ddH 2 O, and added 1 μl lysozyme, incubated at room temperature for 30min. Centrifuge at 20,000×g for 15 minutes at 4°C, take the supernatant and the precipitate respectively, and conduct 12% SDS-PAGE analysis. Select a single clone with an expressed product for strain preservation.
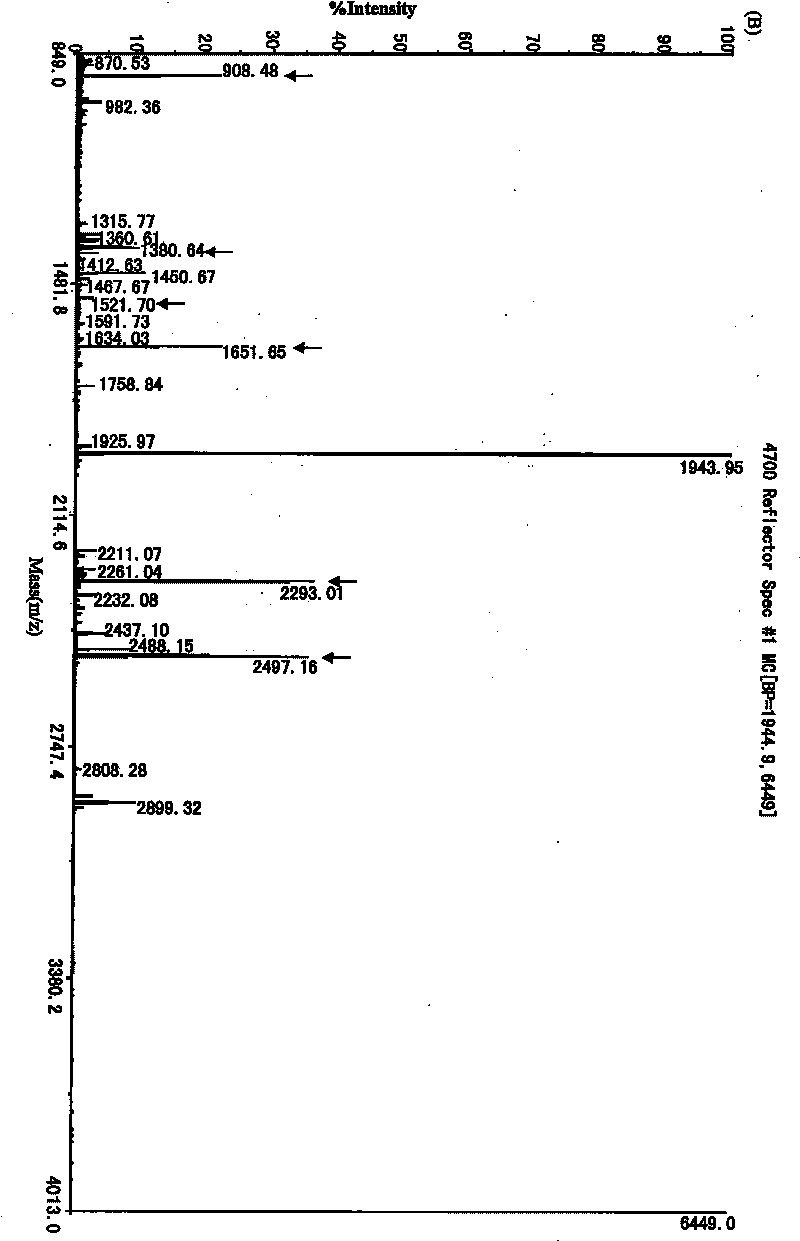
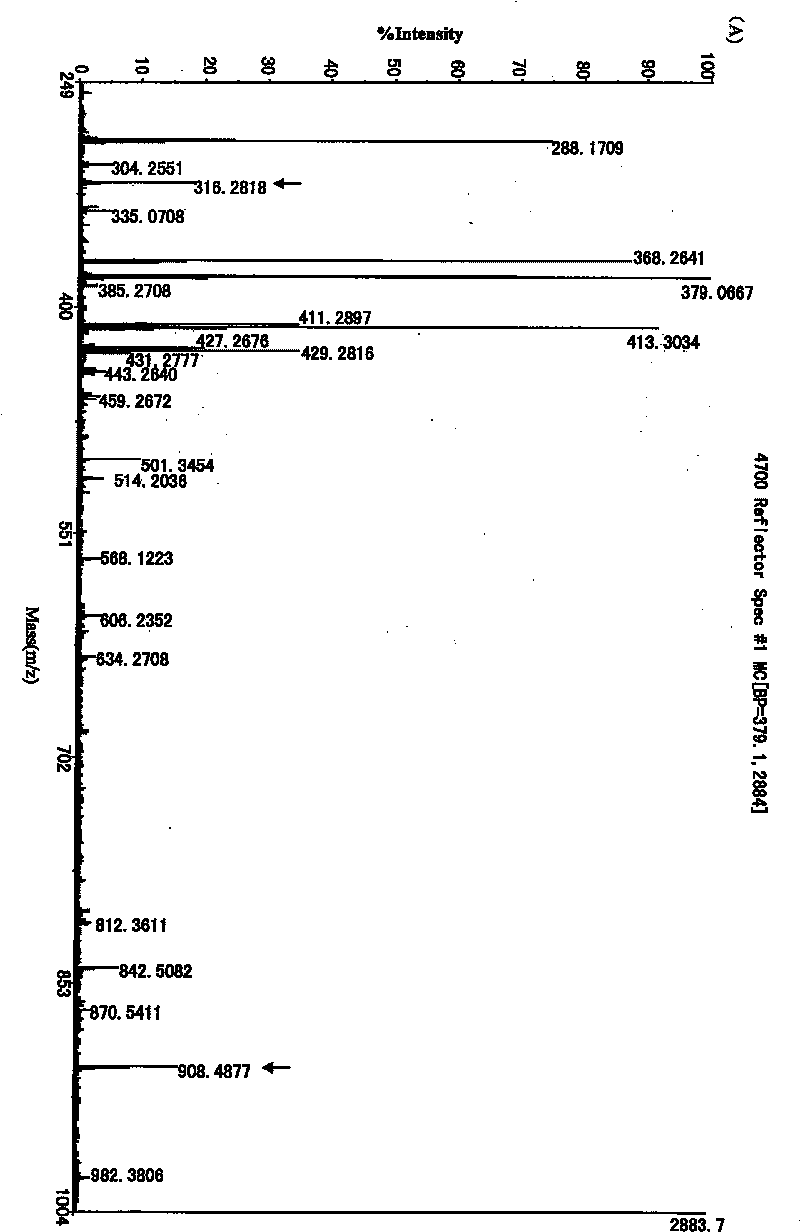
[0050] 2. Analy...
Embodiment 3
[0066] Example 3 Recombinant CVN protein anti-herpes virus activity assay
[0067] Determination of cytotoxicity: MTT method [Xu Shuyun, Bian Rulian, Chen Xiu, editor-in-chief. Pharmacological Experimental Methodology [M]. 2nd Edition. Beijing: People's Medical Publishing House, 1992.] Determination. Add different concentrations of sample diluents to monolayer Vero cells, incubate with 5% CO2 for 48 hours, add 10 μl of 5 mg / ml MTT to each well, continue to incubate for 4 hours in 5% CO2, discard the supernatant, add 200 μl DMSO to each well, and place in the dark at room temperature After 30 minutes, shake the culture plate for about 10 minutes, use an enzyme label reader to measure the color (wavelength 570nm, reference wavelength 630nm), measure the absorbance and calculate the half cytotoxic concentration (50% cytotoxic concentration, TC50) of the sample.
[0068] Determination of anti-HSV-1 activity: 50 μl of samples with different dilutions and 100 TCID50 of HSV-1 virus s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com