Primers for obtaining genes of bovine interferon alpha and preparation method for recombinant bovine interferon alpha
A technology of bovine interferon and alpha gene, which is applied in the preparation method of peptide, interferon, cytokine/lymphokine/interferon, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] A preparation method of recombinant bovine interferon alpha, comprising the following steps:
[0064] a. obtaining the bovine interferon alpha gene, comprising the following steps:
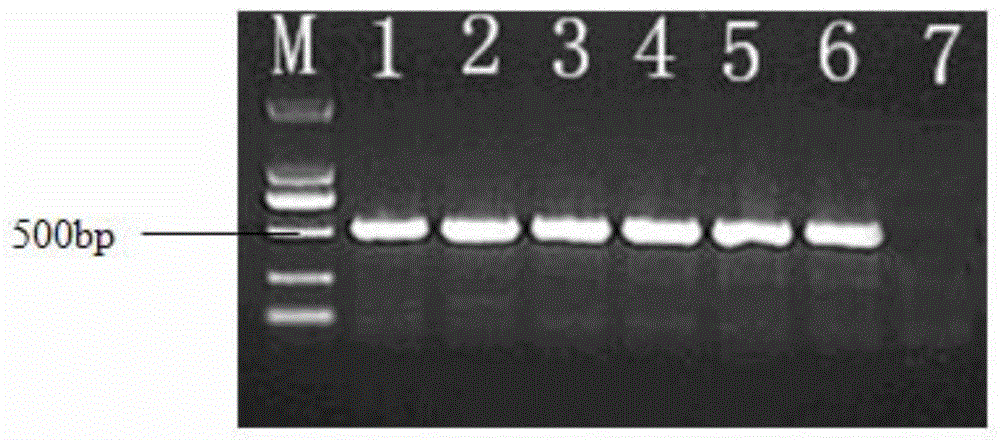
[0065] The RNA extraction kit from TAKARA company was used to extract RNA from bovine liver tissue, and the primers were used for RT-PCR reaction;
[0066] Primer sequence: Upstream: ATA GGA TCC TGC CAC CTGGCCT CAC
[0067] Downstream: ATA AAG CTT TCA GTC CTT TCT CCT G
[0068] The primers have BamHI and HindIII restriction sites respectively;
[0069] The target gene of bovine interferon α is:
[0070] tgccacctgc ctcacaccca cagcctggcc aacaggaggg tcctgatgct cctgcaacaa 60
[0071] ctgagaaggg tctccccttc ctcctgcctg caggacagaa atgacttcga attcctccag 120
[0072] gaggctctgg gtggcagcca gttgcagaag gctcaagcca tctctgtgct ccacgaggtg 180
[0073] accccagcaca ccttccagct cttcagcaca gagggctcgc ccgccacgtg ggacaagagc240
[0074] ctcctggaca agctacgcgc tgcgctggat cagcagctca ctgacctgca agcctgtctg 300
...
Embodiment 2
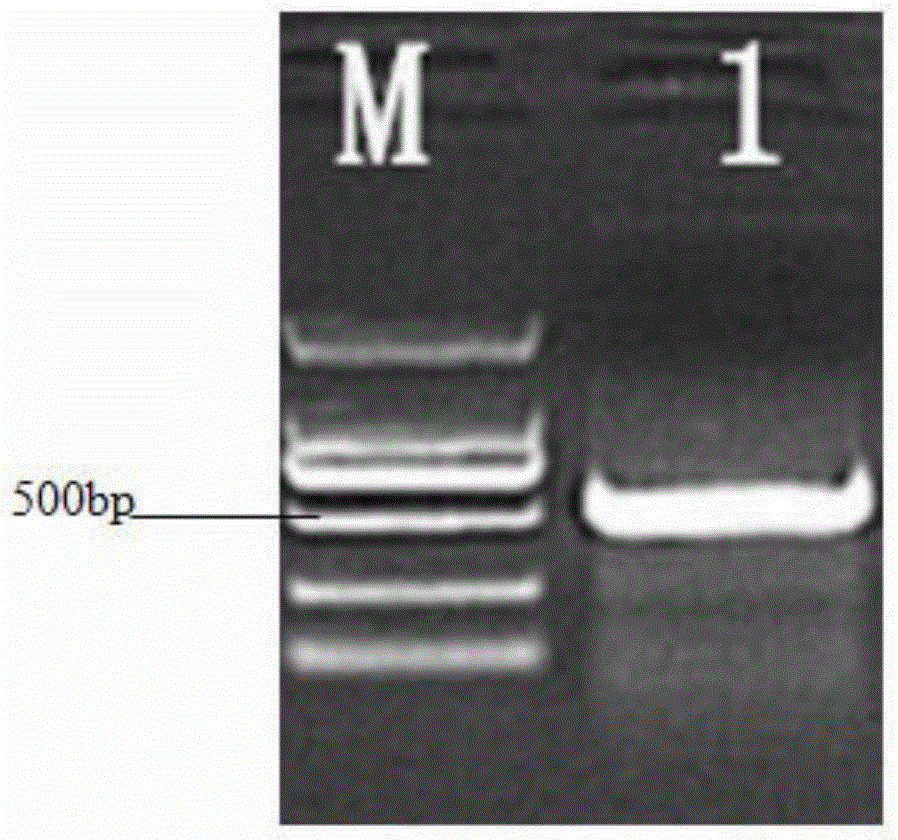
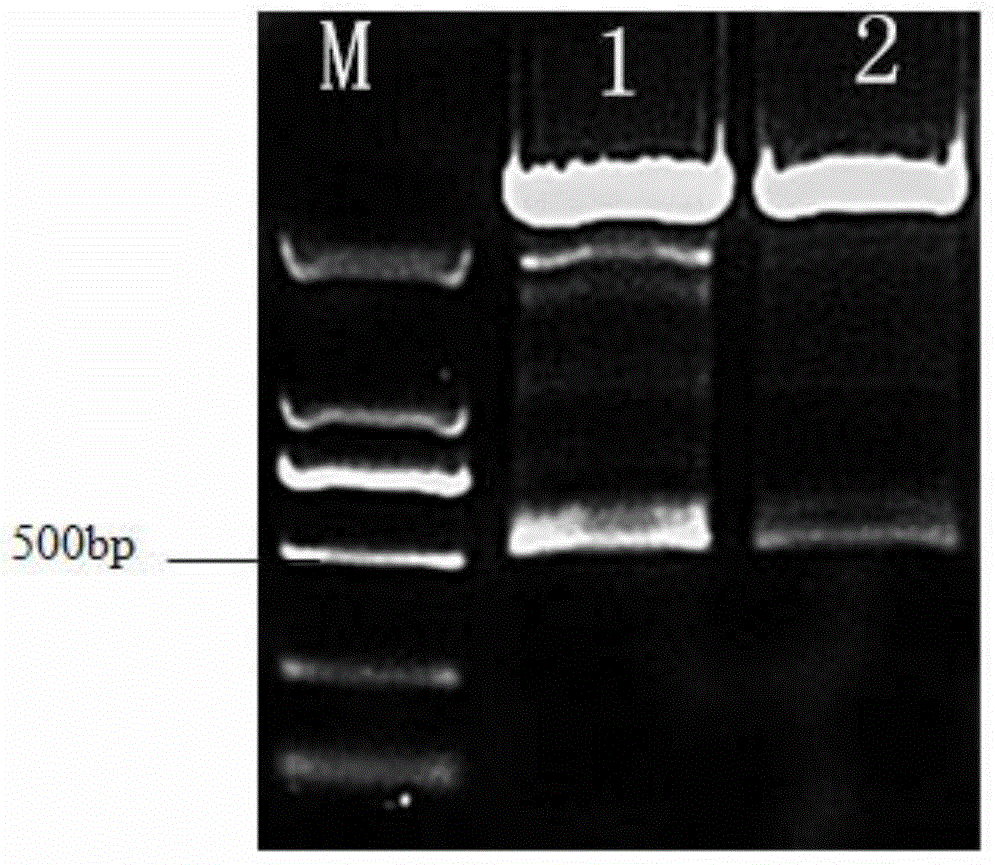
[0101] Identification of Recombinant Bovine Interferon-α:
[0102] Protein Quantitative Detection:
[0103] The Lowry method was used to measure the standard protein of the China Institute for the Control of Food, Drug and Biological Products, and the protein content was determined to be ≥ 1.0 mg / ml.
[0104] SDS-PAGE electrophoresis detection:
[0105] Compared with the empty bacterium control, the recombinant bacterium has a densely stained new target protein band at about 35KD.
[0106] Western Blot results:
[0107] Abcam mouse anti-bovine interferon (1:2000 dilution) was used as polyclonal antibody, and goat anti-mouse IgG-HRP was used as secondary antibody (1:5000 dilution). Recombinant bovine interferon α samples can specifically react with anti-bovine interferon polyclonal antibody, and a specific band appears at about 35KD.
Embodiment 3
[0109] Recombinant bovine interferon α titer detection: According to the microcytopathic inhibition method, using the Hep-2 cell / VSV virus system: culture Hep-2 cells with DMEM nutrient solution on a 96-well microcell culture plate, and place in 5% CO 2 Cultivate in an incubator at 37°C for 24 hours, add different doses of recombinant bovine interferon α, discard after 24 hours, and inoculate 100TCID respectively 50 VSV virus. The results showed that the obtained recombinant bovine interferon α had obvious inhibitory effect on the cytopathic effect caused by VSV. After the untreated cells were inoculated with the virus, the cells became round, shed, and disintegrated. After the obtained recombinant bovine interferon α-treated cells were inoculated with the virus, they were continuously observed under an inverted microscope, and the cell morphology was normal without any pathological changes, and the measured titer was >1.0×10 6 IU / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com