Drug-delivery carrier protein based on cholera toxin CT structure as well as in-vitro construction method and application of drug-delivery carrier protein
A cholera toxin and carrier protein technology, applied in the biological field, can solve the problems of non-expression of foreign proteins, heavy load of dormitory bacteria, and impact on expression, and achieve high protein purity, less assembly interference, and strong repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
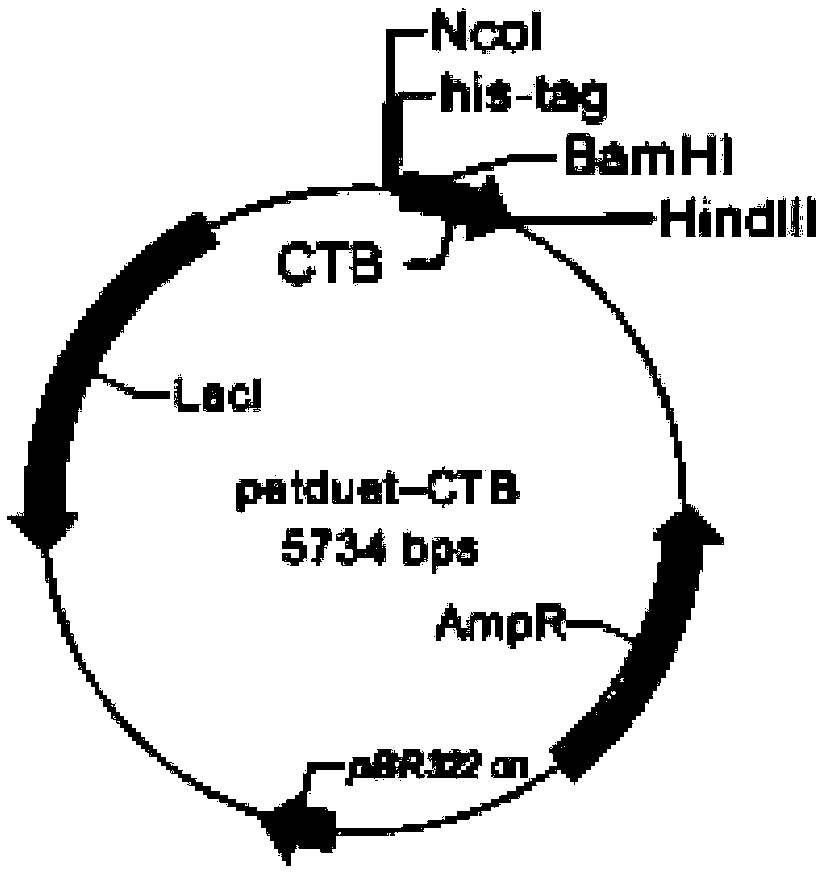
[0051] Gene cloning and expression:
[0052] refer to figure 1 and figure 2 , design primers as follows: CGCGGATCCAACACCTCAAAATATTACTGATTT and CAGCCAACTCAGCTTCCTT, used to amplify the target gene sequence CTB from pet28a-CTB, design primers as follows, CGGAATTCCATATGGTGAGCAAGG and CCGCTCGAGCTGTGGTGGAC, used to amplify from the synthetic gene sequence with NdeI / XhoI Gene sequence EGFP-CTA2-TAT. The amplified CTB was cloned into the multiple cloning site 1 of the vector petduet-1 by BamHI / HindIII double digestion, and EGFP-CTA2-TAT was cloned into The vector pet22b, after preliminary identification by enzyme digestion, was sent to Shanghai Bioengineering Co., Ltd. for sequencing, and the transformants with correct sequencing were screened. The positive clones were marked as petduet-CTB and pet22b-EGFP-CTA2-TAT.
Embodiment 2
[0054] Expression and purification of EGFP-CTA2-TAT soluble protein
[0055] Plasmid pet22b-EGFP-CTA2-TAT introduced into host bacteria , That is, in Escherichia coli BL21 (DE3), and realize the expression; the plasmid that has been transformed into pet22b-EGFP-CTA2-TAT is expanded and cultured at a ratio of 1:100 in 2L LB medium rich in 100ug / mL ampicillin, at 37 degrees After culturing in a shaker at 200 rpm until the OD value was 2, IPTG with a final concentration of 1 mM was added to induce the culture for 8 hours. The culture was divided into 300ml centrifuge tubes, centrifuged at 10000rpm, 4°C for 30min, the culture medium was discarded, the bacteria were collected and washed 3 times with 200ml PBS containing 2% tritonx-100, and the bacteria were collected. Add 4ml of PBS containing 2% tritonx-100 to every 100ml of the original culture, and add lysozyme (purchased from Shanghai Sangong) at a final concentration of 200ug / ml, and freeze and thaw repeatedly at -20°C for 3 ...
Embodiment 3
[0057] Purification and Refolding of Cholera Toxin B Subunit Inclusion Body Protein
[0058] The plasmid petduet-CTB was introduced into the host strain BL21(DE3) and expressed. After the plasmid petduet-CTB was transformed into Escherichia coli BL21(DE3), the steps before repeated freezing and thawing were consistent with the steps for obtaining the EGFP-CTA2-TAT protein. After repeated freezing and thawing three times, transfer to a centrifuge, centrifuge at 4 degrees, 10000rpm for 30min, and collect the precipitate. The precipitate was washed three times with buffer I (pH=8.0, 100 mM NaCl, 2% tritonx-100, 20 mM tris-HCl, 5 mM EDTA), followed by successive washing with buffer I of 2M, 4M, 6M, and 8M urea, and the supernatant was collected. SDS PAGE was used to detect whether the CTB monomer in the supernatant was pure. The CTB supernatant with high purity was screened for renaturation. CTB supernatant containing urea was dialyzed and refolded with buffer II (buffer I, 1 mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com